Ernest Rutherford Model
Siužetinės Linijos Tekstas
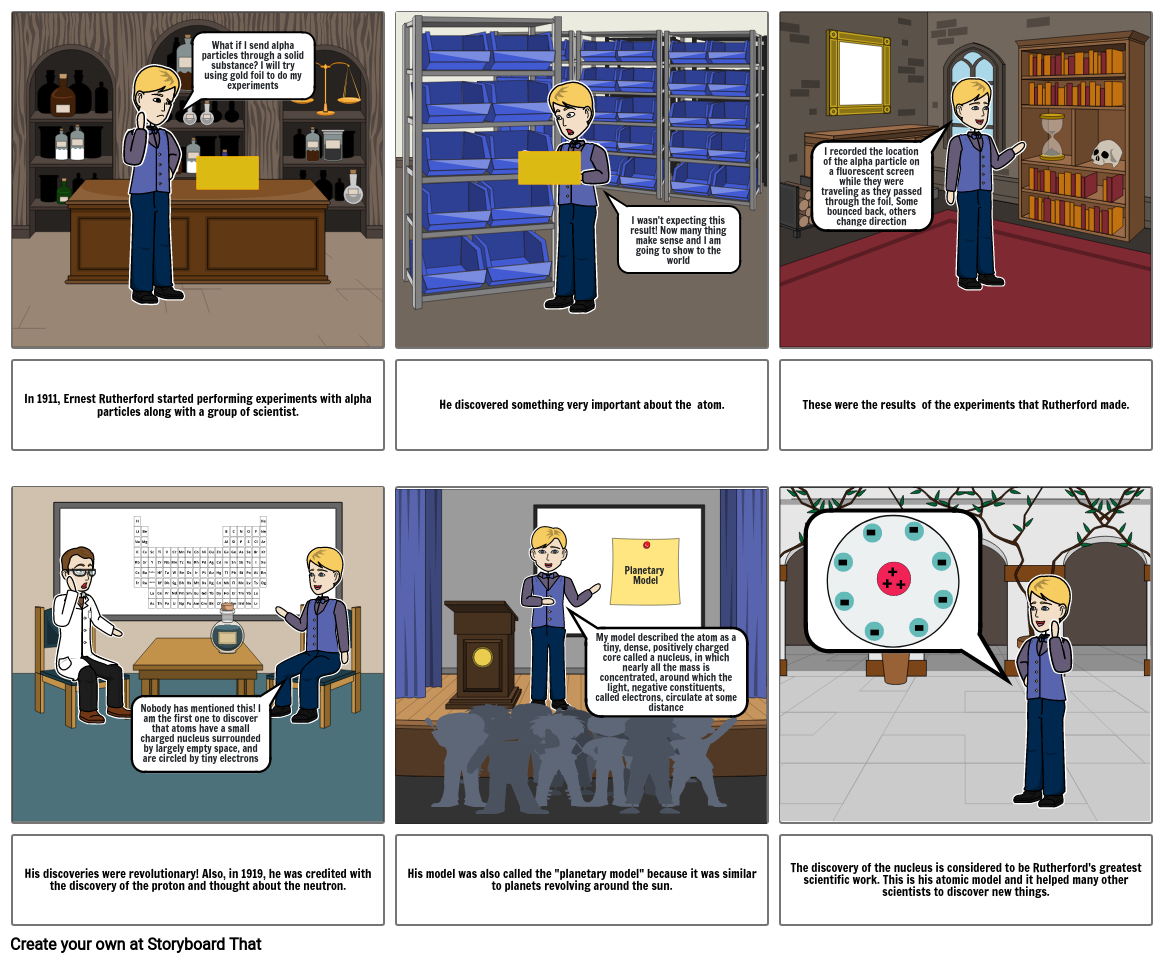
- What if I send alpha particles through a solid substance? I will try using gold foil to do my experiments
- I wasn't expecting this result! Now many thing make sense and I am going to show to the world
- I recorded the location of the alpha particle on a fluorescent screen while they were traveling as they passed through the foil. Some bounced back, others change direction
- In 1911, Ernest Rutherford started performing experiments with alpha particles along with a group of scientist.
- Nobody has mentioned this! I am the first one to discover that atoms have a small charged nucleus surrounded by largely empty space, and are circled by tiny electrons
- He discovered something very important about the atom.
- My model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called electrons, circulate at some distance
- Planetary Model
- These were the results of the experiments that Rutherford made.
- His discoveries were revolutionary! Also, in 1919, he was credited with the discovery of the proton and thought about the neutron.
- His model was also called the "planetary model" because it was similar to planets revolving around the sun.
- The discovery of the nucleus is considered to be Rutherford's greatest scientific work. This is his atomic model and it helped many other scientists to discover new things.
Sukurta daugiau nei 30 milijonų siužetinių lentelių

