Unknown Story
Siužetinės Linijos Tekstas
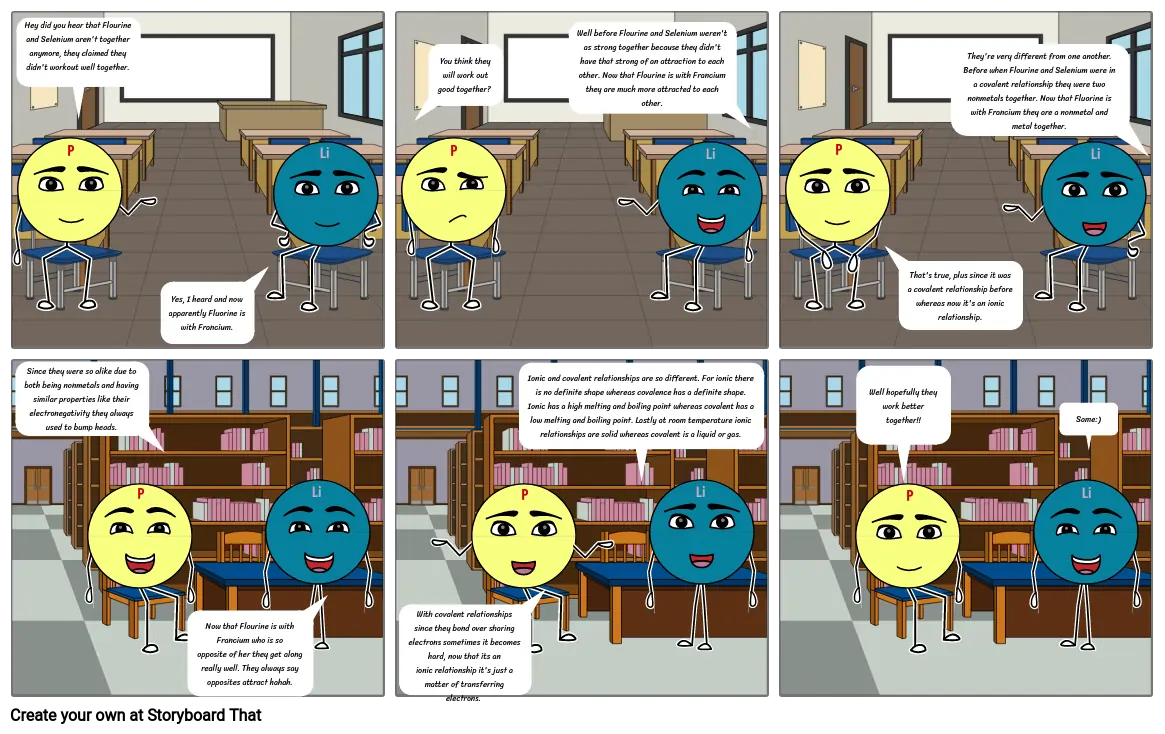
- Hey did you hear that Flourine and Selenium aren't together anymore, they claimed they didn't workout well together.
- P
- Yes, I heard and now apparently Fluorine is with Francium.
- Li
- You think they will work out good together?
- P
- Well before Flourine and Selenium weren't as strong together because they didn't have that strong of an attraction to each other. Now that Flourine is with Francium they are much more attracted to each other.
- Li
- P
- That's true, plus since it was a covalent relationship before whereas now it's an ionic relationship.
- They're very different from one another. Before when Flourine and Selenium were in a covalent relationship they were two nonmetals together. Now that Fluorine is with Francium they are a nonmetal and metal together.
- Li
- Since they were so alike due to both being nonmetals and having similar properties like their electronegativity they always used to bump heads.
- P
- Now that Flourine is with Francium who is so opposite of her they get along really well. They always say opposites attract hahah.
- Li
- With covalent relationships since they bond over sharing electrons sometimes it becomes hard, now that its an ionic relationship it's just a matter of transferring electrons.
- P
- Ionic and covalent relationships are so different. For ionic there is no definite shape whereas covalence has a definite shape. Ionic has a high melting and boiling point whereas covalent has a low melting and boiling point. Lastly at room temperature ionic relationships are solid whereas covalent is a liquid or gas.
- Li
- Well hopefully they work better together!!
- P
- Same:)
- Li
Sukurta daugiau nei 30 milijonų siužetinių lentelių

