Ionic Bonding
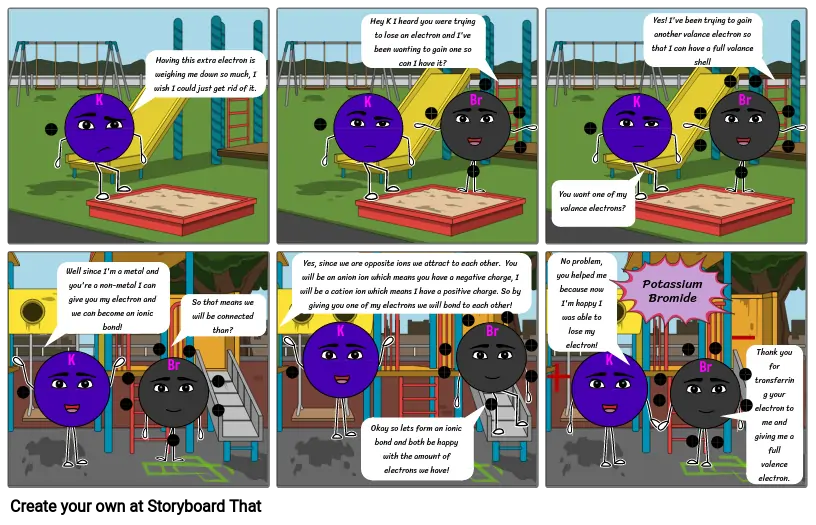
Siužetinės Linijos Tekstas
- K
- Having this extra electron is weighing me down so much, I wish I could just get rid of it.
- K
- Hey K I heard you were trying to lose an electron and I've been wanting to gain one so can I have it?
- Br
- .
- You want one of my valance electrons?
- K
- Yes! I've been trying to gain another valance electron so that I can have a full valance shell
- Br
- Well since I'm a metal and you're a non-metal I can give you my electron and we can become an ionic bond!
- K
- Br
- So that means we will be connected than?
- Yes, since we are opposite ions we attract to each other. You will be an anion ion which means you have a negative charge, I will be a cation ion which means I have a positive charge. So by giving you one of my electrons we will bond to each other!
- K
- Okay so lets form an ionic bond and both be happy with the amount of electrons we have!
- Br
- No problem, you helped me because now I'm happy I was able to lose my electron!
- K
- Potassium Bromide
- Br
- Thank you for transferring your electron to me and giving me a full valence electron.
Sukurta daugiau nei 30 milijonų siužetinių lentelių
Nereikia Atsisiuntimų, Nereikia Kredito Kortelės ir Nereikia Prisijungti!

