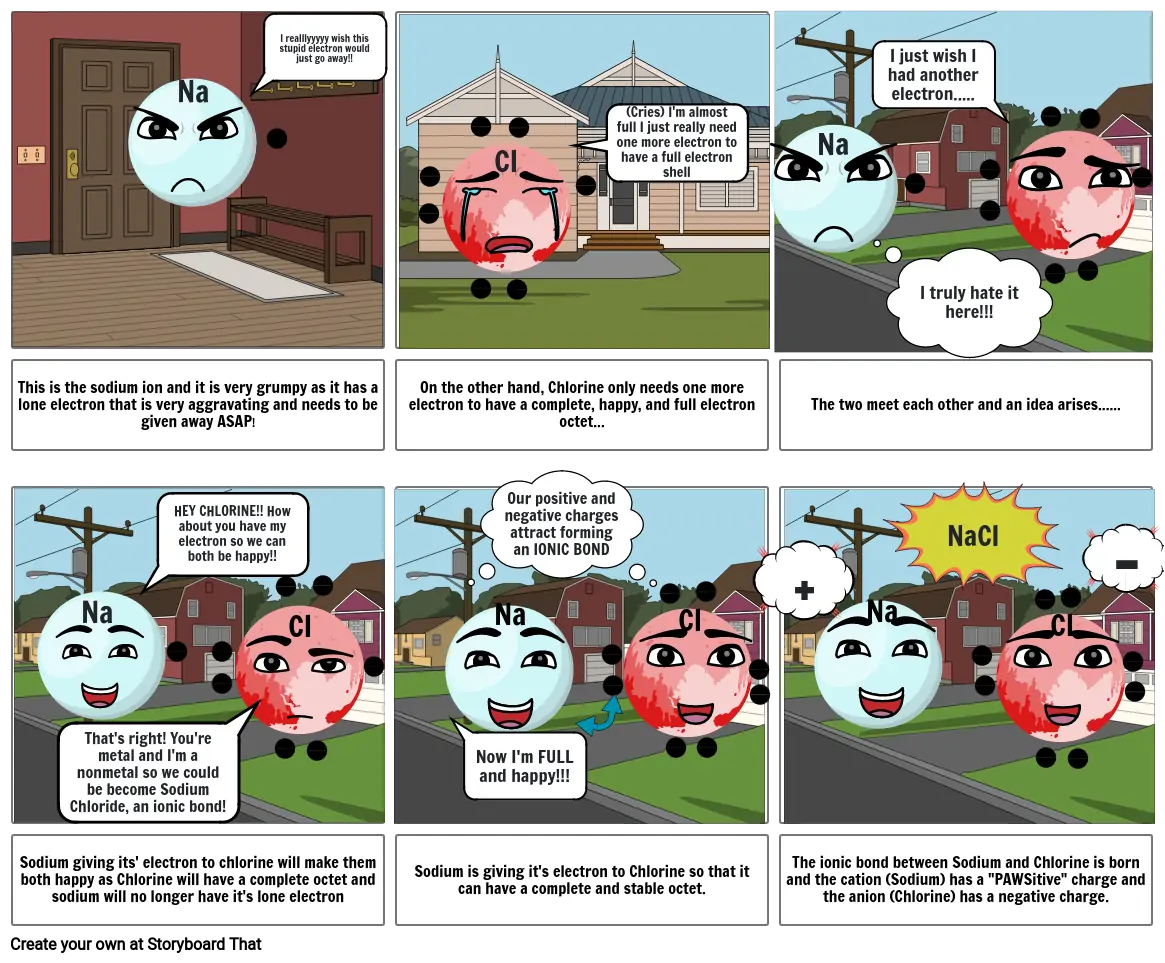
Siužetinės Linijos Tekstas
- Na
- I realllyyyyy wish this stupid electron would just go away!!
- Cl
- (Cries) I'm almost full I just really need one more electron to have a full electron shell
- Na
- I just wish I had another electron.....
- I truly hate it here!!!
- This is the sodium ion and it is very grumpy as it has a lone electron that is very aggravating and needs to be given away ASAP!
- Na
- HEY CHLORINE!! How about you have my electron so we can both be happy!!
- Cl
- On the other hand, Chlorine only needs one more electron to have a complete, happy, and full electron octet...
- Our positive and negative charges attract forming an IONIC BOND
- Na
- Cl
- +
- The two meet each other and an idea arises......
- Na
- NaCl
- Cl
- -
- Sodium giving its' electron to chlorine will make them both happy as Chlorine will have a complete octet and sodium will no longer have it's lone electron
- That's right! You're metal and I'm a nonmetal so we could be become Sodium Chloride, an ionic bond!
- Sodium is giving it's electron to Chlorine so that it can have a complete and stable octet.
- Now I'm FULL and happy!!!
- The ionic bond between Sodium and Chlorine is born and the cation (Sodium) has a "PAWSitive" charge and the anion (Chlorine) has a negative charge.
Sukurta daugiau nei 30 milijonų siužetinių lentelių

