CHEMISTRY ALLOTROPES OF CARBON
Siužetinės Linijos Tekstas
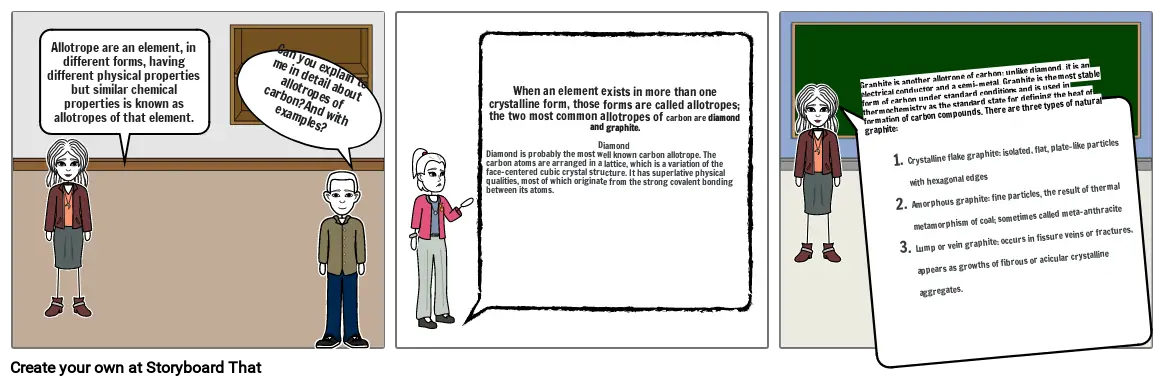
- Allotrope are an element, in different forms, having different physical properties but similar chemical properties is known as allotropes of that element.
- Can you explain to me in detail about allotropes of carbon?And with examples?
- When an element exists in more than one crystalline form, those forms are called allotropes; the two most common allotropes of carbon are diamond and graphite.DiamondDiamond is probably the most well known carbon allotrope. The carbon atoms are arranged in a lattice, which is a variation of the face-centered cubic crystal structure. It has superlative physical qualities, most of which originate from the strong covalent bonding between its atoms.
- Graphite is another allotrope of carbon; unlike diamond, it is an electrical conductor and a semi-metal. Graphite is the most stable form of carbon under standard conditions and is used in thermochemistry as the standard state for defining the heat of formation of carbon compounds. There are three types of natural graphite:Crystalline flake graphite: isolated, flat, plate-like particles with hexagonal edgesAmorphous graphite: fine particles, the result of thermal metamorphism of coal; sometimes called meta-anthraciteLump or vein graphite: occurs in fissure veins or fractures, appears as growths of fibrous or acicular crystalline aggregates.
Sukurta daugiau nei 30 milijonų siužetinių lentelių

