Reality show
Siužetinės Linijos Tekstas
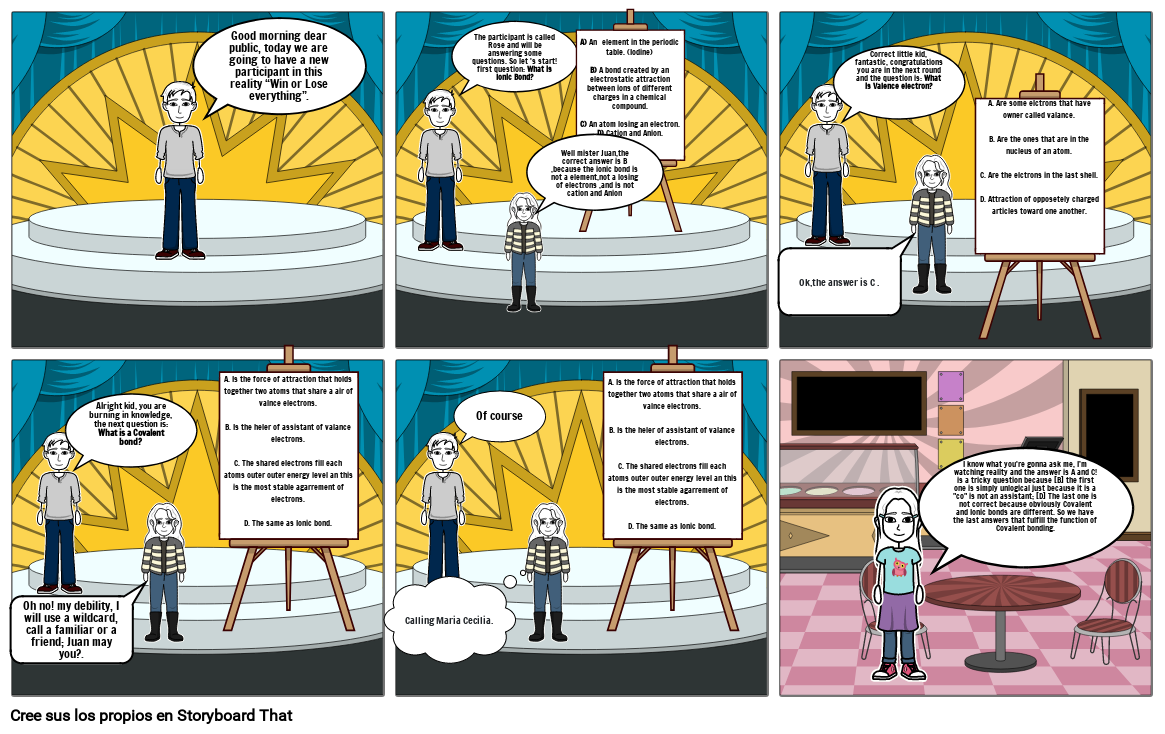
- Good morning dear public, today we are going to have a new participant in this reality “Win or Lose everything”.
- A. Is the force of attraction that holds together two atoms that share a air of valnce electrons.B. Is the heler of assistant of valance electrons.C. The shared electrons fill each atoms outer outer energy level an this is the most stable agarrement of electrons.D. The same as Ionic bond.
- The participant is called Rose and will be answering some questions. So let 's start! first question: What is Ionic Bond?
- Well mister Juan,the correct answer is B ,because the ionic bond is not a element,not a losing of electrons ,and is not cation and Anion
- A) An element in the periodic table. (Iodine)B) A bond created by an electrostatic attraction between ions of different charges in a chemical compound.C) An atom losing an electron. D) Cation and Anion.
- A. Is the force of attraction that holds together two atoms that share a air of valnce electrons.B. Is the heler of assistant of valance electrons.C. The shared electrons fill each atoms outer outer energy level an this is the most stable agarrement of electrons.D. The same as Ionic bond.
- Ok,the answer is C .
- Correct little kid, fantastic, congratulations you are in the next round and the question is: What is Valence electron?
- A. Are some elctrons that have owner called valance.B. Are the ones that are in the nucleus of an atom.C. Are the elctrons in the last shell.D. Attraction of opposetely charged articles toward one another.
- Oh no! my debility, I will use a wildcard, call a familiar or a friend; Juan may you?.
- Alright kid, you are burning in knowledge, the next question is: What is a Covalent bond?
- Calling Maria Cecilia.
- Of course
- I know what you're gonna ask me, I’m watching reality and the answer is A and C! is a tricky question because [B] the first one is simply unlogical just because it is a "co" is not an assistant; [D] The last one is not correct because obviously Covalent and Ionic bonds are different. So we have the last answers that fulfill the function of Covalent bonding.
Sukurta daugiau nei 30 milijonų siužetinių lentelių

