part 3
Siužetinės Linijos Tekstas
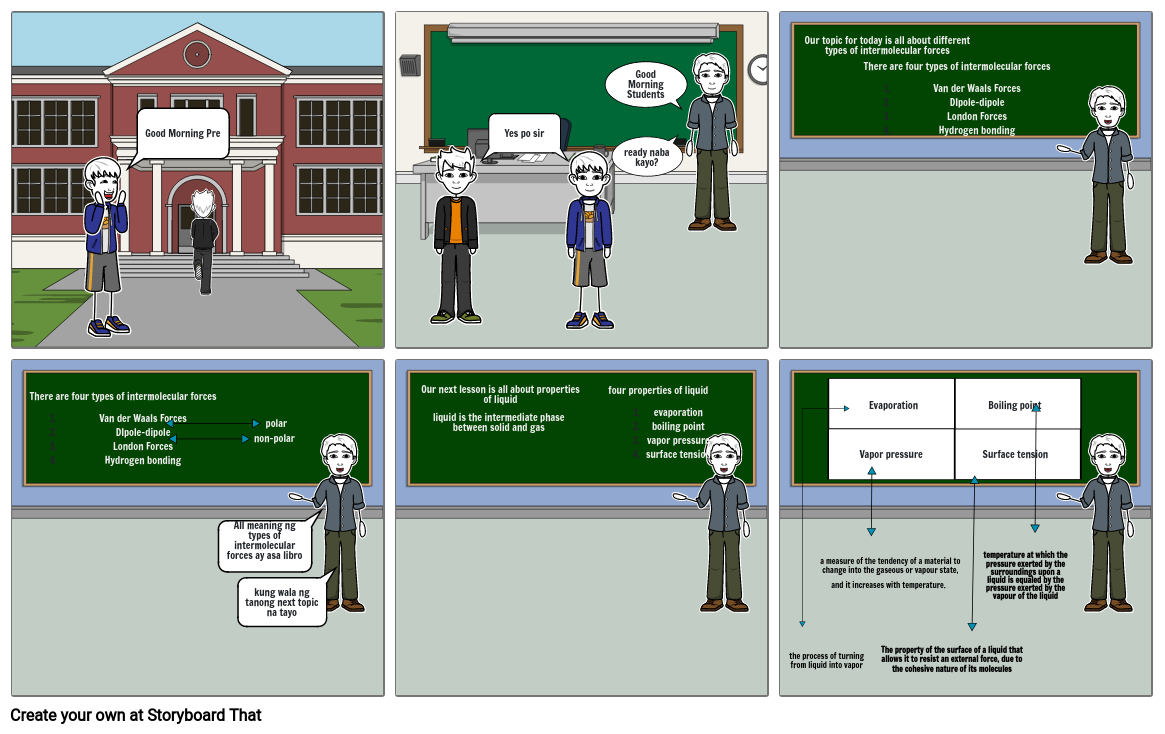
- Good Morning Pre
- Yes po sir
- Good Morning Students
- ready naba kayo?
- Our topic for today is all about different types of intermolecular forces
- There are four types of intermolecular forcesVan der Waals ForcesDIpole-dipoleLondon ForcesHydrogen bonding
- There are four types of intermolecular forcesVan der Waals ForcesDIpole-dipoleLondon ForcesHydrogen bonding
- All meaning ng types of intermolecular forces ay asa libro
- non-polar
- polar
- kung wala ng tanong next topic na tayo
- Our next lesson is all about properties of liquid
- liquid is the intermediate phase between solid and gas
- four properties of liquidevaporationboiling pointvapor pressuresurface tension
- the process of turning from liquid into vapor
- a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with temperature..
- Vapor pressure
- Evaporation
- The property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of its molecules
- Boiling point
- Surface tension
- temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid
Sukurta daugiau nei 30 milijonų siužetinių lentelių

