gen chem
Siužetinės Linijos Tekstas
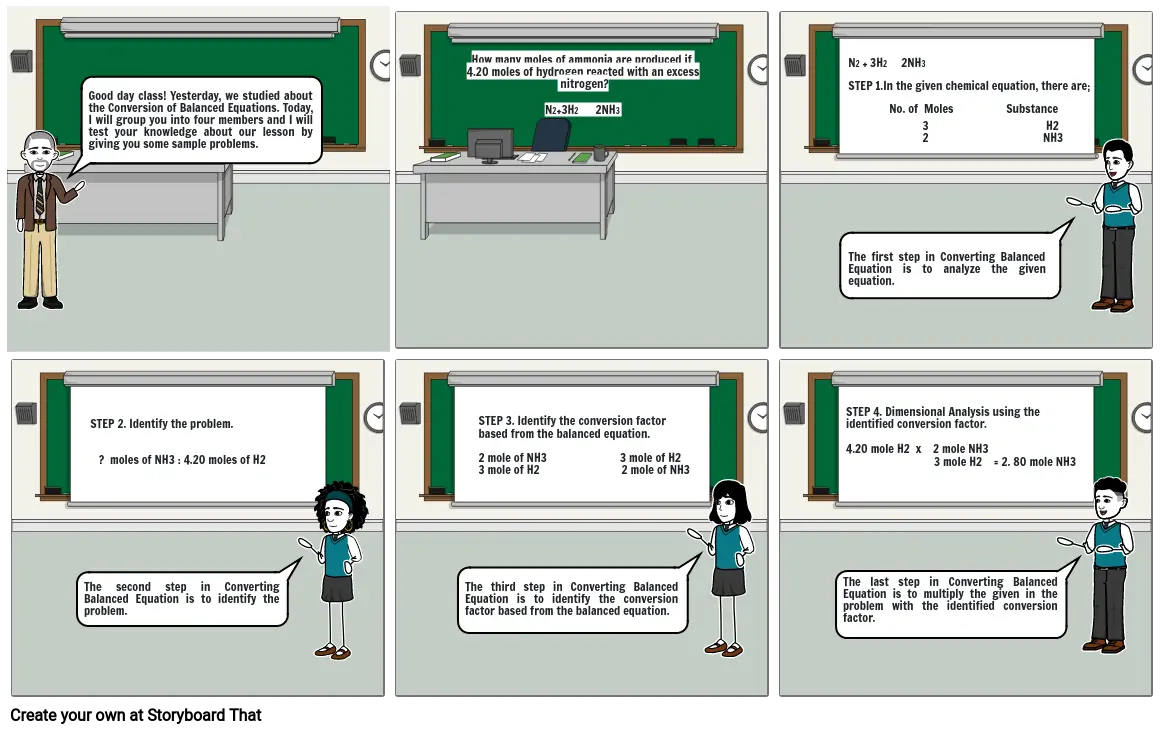
- Good day class! Yesterday, we studied about the Conversion of Balanced Equations. Today, I will group you into four members and I will test your knowledge about our lesson by giving you some sample problems.
- How many moles of ammonia are produced if 4.20 moles of hydrogen reacted with an excess nitrogen?N2+3H2 2NH3
-
- N2 + 3H2 2NH3STEP 1.In the given chemical equation, there are; No. of Moles Substance
- The first step in Converting Balanced Equation is to analyze the given equation.
- 3 H2 2 NH3
- The second step in Converting Balanced Equation is to identify the problem.
- STEP 2. Identify the problem. ? moles of NH3 : 4.20 moles of H2
- The third step in Converting Balanced Equation is to identify the conversion factor based from the balanced equation.
- STEP 3. Identify the conversion factor based from the balanced equation.2 mole of NH3 3 mole of H23 mole of H2 2 mole of NH3
- The last step in Converting Balanced Equation is to multiply the given in the problem with the identified conversion factor.
- STEP 4. Dimensional Analysis using the identified conversion factor.4.20 mole H2 x 2 mole NH3 3 mole H2 = 2. 80 mole NH3
Sukurta daugiau nei 30 milijonų siužetinių lentelių

