Siužetinės Linijos Tekstas
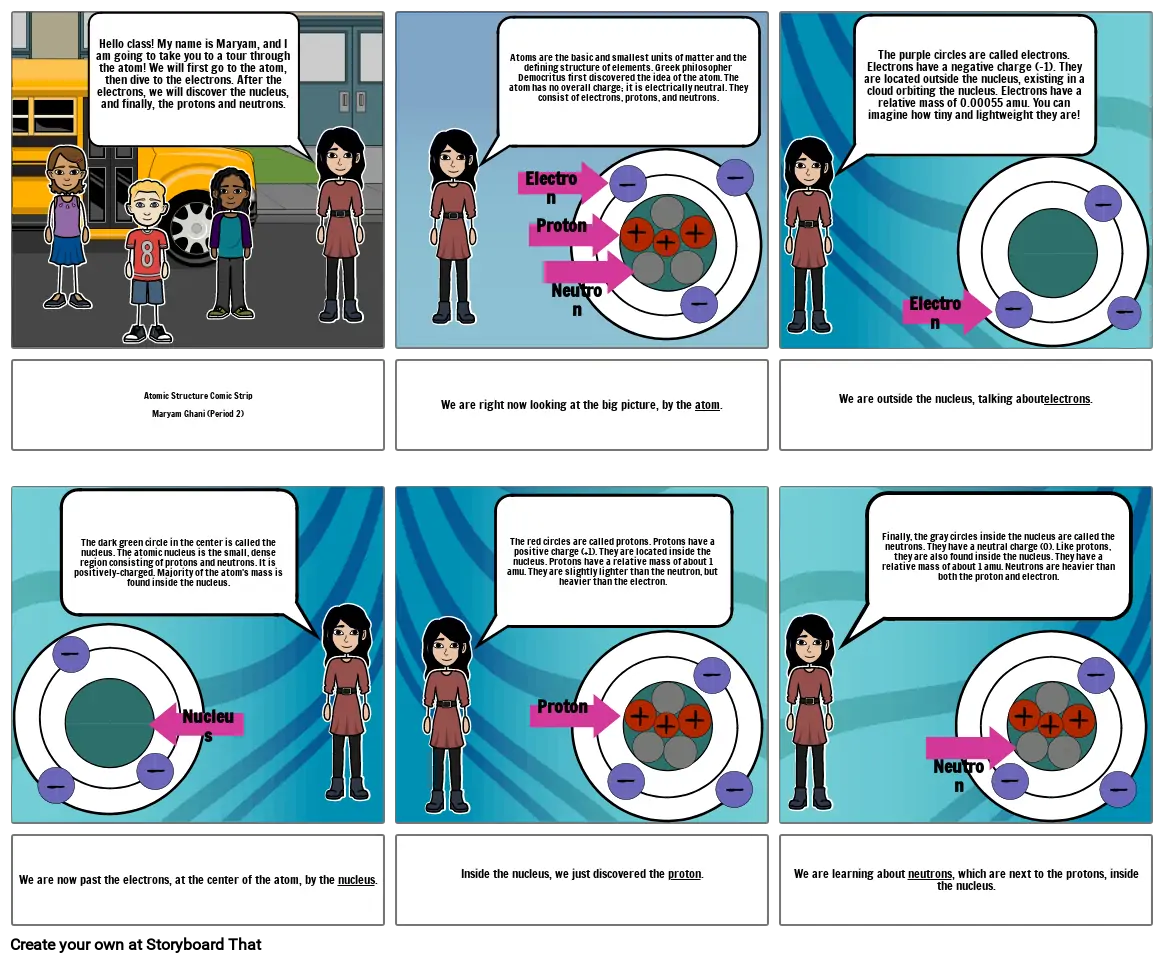
- Hello class! My name is Maryam, and I am going to take you to a tour through the atom! We will first go to the atom, then dive to the electrons. After the electrons, we will discover the nucleus, and finally, the protons and neutrons.
- Atoms are the basic and smallest units of matter and the defining structure of elements. Greek philosopher Democritus first discovered the idea of the atom. The atom has no overall charge; it is electrically neutral. They consist of electrons, protons, and neutrons.
- Electron
- Proton
- Neutron
- The purple circles are called electrons. Electrons have a negative charge (-1). They are located outside the nucleus, existing in a cloud orbiting the nucleus. Electrons have a relative mass of 0.00055 amu. You can imagine how tiny and lightweight they are!
- Electron
- Atomic Structure Comic StripMaryam Ghani (Period 2)
- The dark green circle in the center is called the nucleus. The atomic nucleus is the small, dense region consisting of protons and neutrons. It is positively-charged. Majority of the atom's mass is found inside the nucleus.
- We are right now looking at the big picture, by the atom.
- The red circles are called protons. Protons have a positive charge (+1). They are located inside the nucleus. Protons have a relative mass of about 1 amu. They are slightly lighter than the neutron, but heavier than the electron.
- Proton
- We are outside the nucleus, talking aboutelectrons.
- Finally, the gray circles inside the nucleus are called the neutrons. They have a neutral charge (0). Like protons, they are also found inside the nucleus. They have a relative mass of about 1 amu. Neutrons are heavier than both the proton and electron.
- We are now past the electrons, at the center of the atom, by the nucleus.
- Nucleus
- Inside the nucleus, we just discovered the proton.
- We are learning about neutrons, which are next to the protons, inside the nucleus.
- Neutron
Sukurta daugiau nei 30 milijonų siužetinių lentelių

