Ocean Acidfication
Siužetinės Linijos Aprašymas
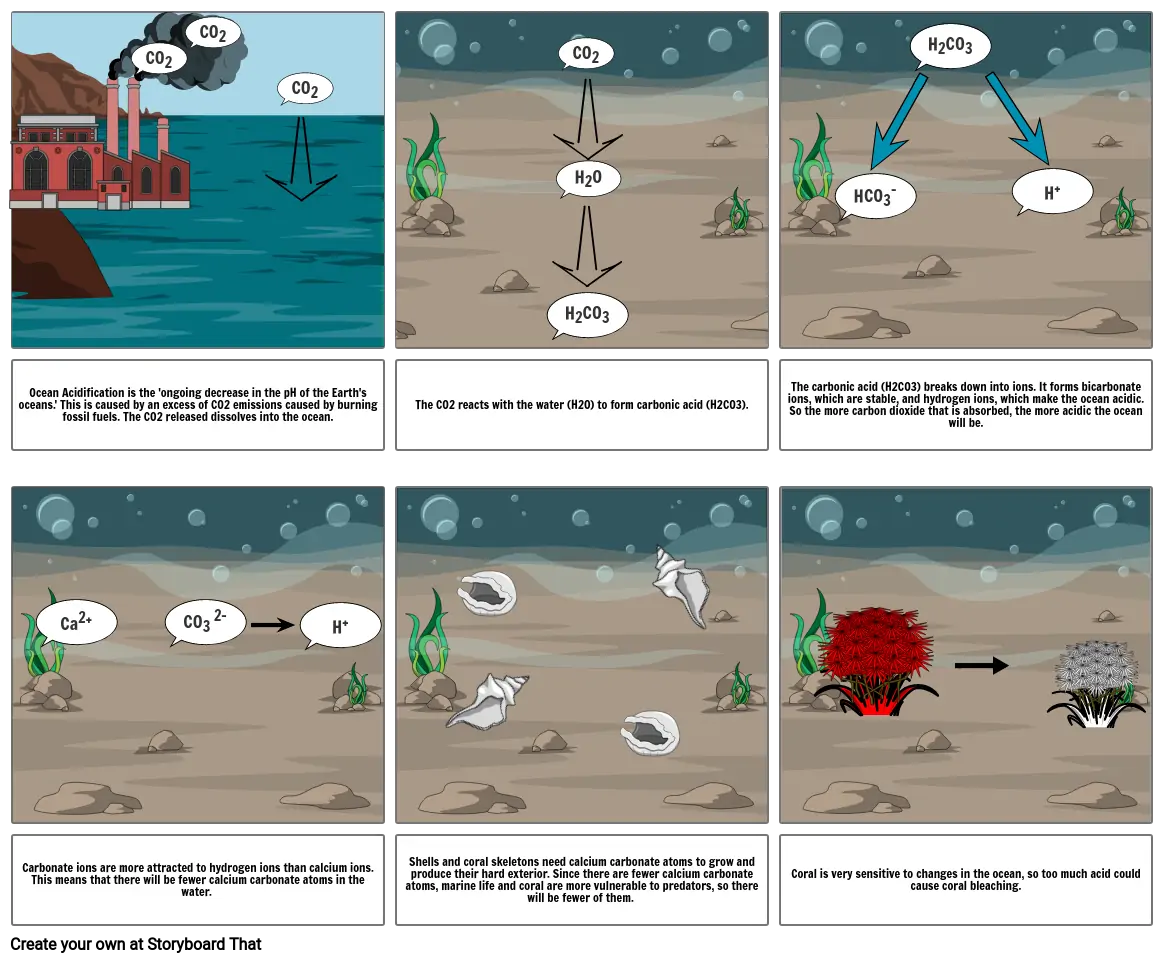
Shows hao the process of Ocean Acidfication works
Siužetinės Linijos Tekstas
- CO2
- CO2
- CO2
- H2CO3
- H2O
- CO2
- HCO3-
- H2CO3
- H+
- Ocean Acidification is the 'ongoing decrease in the pH of the Earth's oceans.' This is caused by an excess of CO2 emissions caused by burning fossil fuels. The CO2 released dissolves into the ocean.
- Ca2+
- CO3 2-
- H+
- The CO2 reacts with the water (H2O) to form carbonic acid (H2CO3).
- The carbonic acid (H2CO3) breaks down into ions. It forms bicarbonate ions, which are stable, and hydrogen ions, which make the ocean acidic. So the more carbon dioxide that is absorbed, the more acidic the ocean will be.
- Carbonate ions are more attracted to hydrogen ions than calcium ions. This means that there will be fewer calcium carbonate atoms in the water.
- Shells and coral skeletons need calcium carbonate atoms to grow and produce their hard exterior. Since there are fewer calcium carbonate atoms, marine life and coral are more vulnerable to predators, so there will be fewer of them.
- Coral is very sensitive to changes in the ocean, so too much acid could cause coral bleaching.
Sukurta daugiau nei 30 milijonų siužetinių lentelių

