Charles Law
Siužetinės Linijos Tekstas
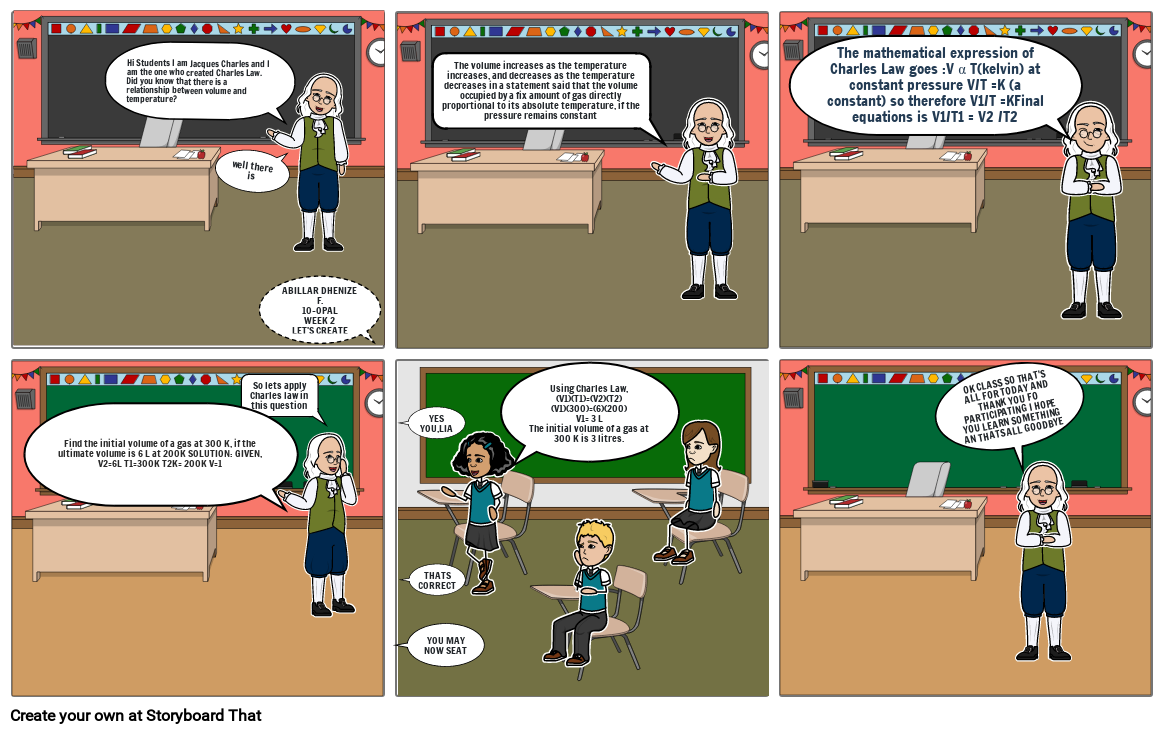
- Hi Students I am Jacques Charles and I am the one who created Charles Law. Did you know that there is a relationship between volume and temperature?
- well there is
- ABILLAR DHENIZE F.10-OPALWEEK 2 LET'S CREATE
- The volume increases as the temperature increases, and decreases as the temperature decreases in a statement said that the volume occupied by a fix amount of gas directly proportional to its absolute temperature, if the pressure remains constant
- The mathematical expression of Charles Law goes :V α T(kelvin) at constant pressure V/T =K (a constant) so therefore V1/T =KFinal equations is V1/T1 = V2 /T2
- Find the initial volume of a gas at 300 K, if the ultimate volume is 6 L at 200K SOLUTION: GIVEN, V2=6L T1=300K T2K= 200K V=1
- So lets apply Charles law in this question
- YOU MAY NOW SEAT
- YES YOU,LIA
- THATS CORRECT
- Using Charles Law,(V1)(T1)=(V2)(T2)(V1)(300)=(6)(200)V1= 3 LThe initial volume of a gas at 300 K is 3 litres.
- OK CLASS SO THAT'S ALL FOR TODAY AND THANK YOU FO PARTICIPATING I HOPE YOU LEARN SOMETHING AN THATS ALL GOODBYE
Sukurta daugiau nei 30 milijonų siužetinių lentelių

