The History of Periodic Table
Siužetinės Linijos Tekstas
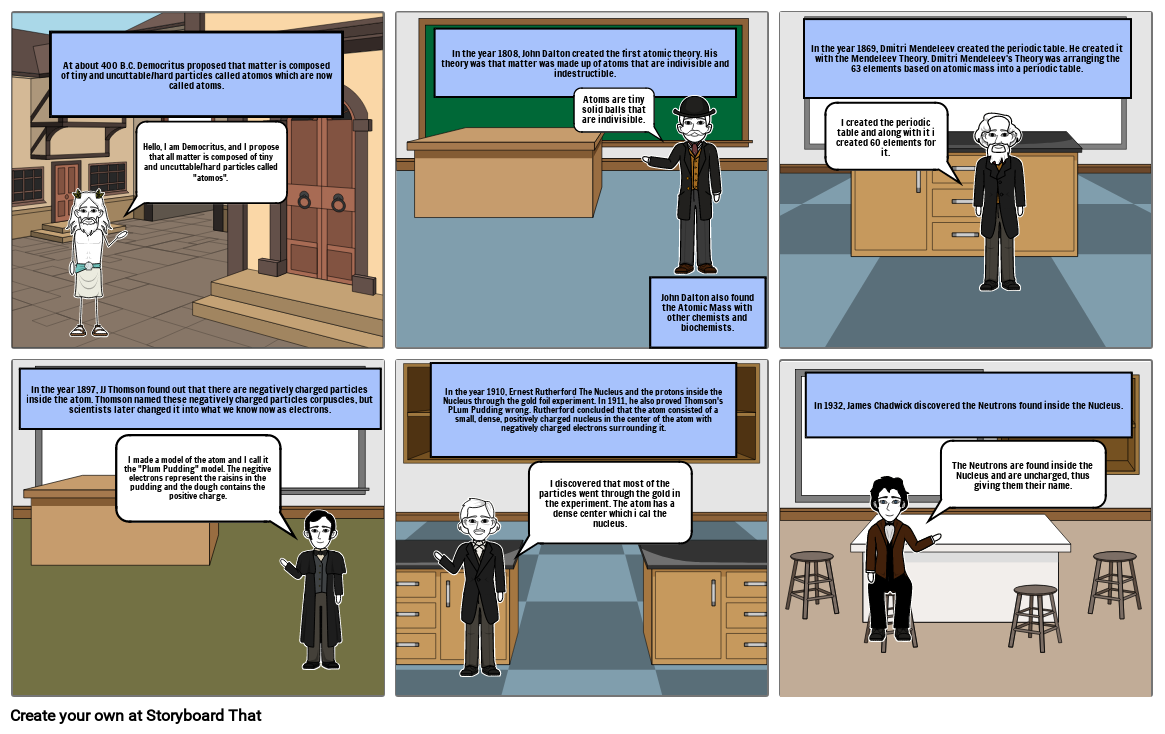
- At about 400 B.C. Democritus proposed that matter is composed of tiny and uncuttable/hard particles called atomos which are now called atoms.
- Hello, I am Democritus, and I propose that all matter is composed of tiny and uncuttable/hard particles called "atomos".
- In the year 1808, John Dalton created the first atomic theory. His theory was that matter was made up of atoms that are indivisible and indestructible.
- Atoms are tiny solid balls that are indivisible.
- John Dalton also found the Atomic Mass with other chemists and biochemists.
- In the year 1869, Dmitri Mendeleev created the periodic table. He created it with the Mendeleev Theory. Dmitri Mendeleev's Theory was arranging the 63 elements based on atomic mass into a periodic table.
- I created the periodic table and along with it i created 60 elements for it.
- In the year 1897, JJ Thomson found out that there are negatively charged particles inside the atom. Thomson named these negatively charged particles corpuscles, but scientists later changed it into what we know now as electrons.
- I made a model of the atom and I call it the "Plum Pudding" model. The negitive electrons represent the raisins in the pudding and the dough contains the positive charge.
- In the year 1910, Ernest Rutherford The Nucleus and the protons inside the Nucleus through the gold foil experiment. In 1911, he also proved Thomson's PLum Pudding wrong. Rutherford concluded that the atom consisted of a small, dense, positively charged nucleus in the center of the atom with negatively charged electrons surrounding it.
- I discovered that most of the particles went through the gold in the experiment. The atom has a dense center which i cal the nucleus.
- In 1932, James Chadwick discovered the Neutrons found inside the Nucleus.
- The Neutrons are found inside the Nucleus and are uncharged, thus giving them their name.
Sukurta daugiau nei 30 milijonų siužetinių lentelių

