Chemical Bonding
Siužetinės Linijos Tekstas
- H H H H
- Na Na
- Na Na
- Cl Cl
- + +
- - -
- C C
- H H
- H H
- C C
- Cl Cl
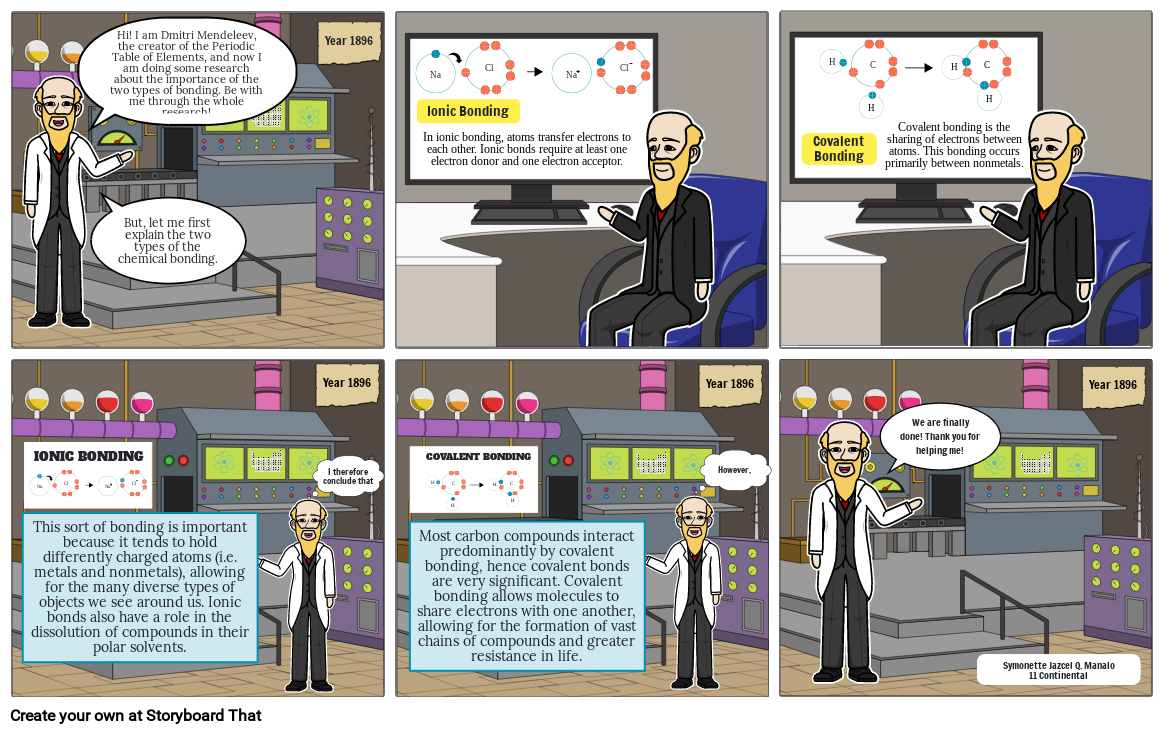
- Hi! I am Dmitri Mendeleev, the creator of the Periodic Table of Elements, and now I am doing some research about the importance of the two types of bonding. Be with me through the whole research!
- But, let me first explain the two types of the chemical bonding.
- Year 1896
- In ionic bonding, atoms transfer electrons to each other. Ionic bonds require at least one electron donor and one electron acceptor.
- Ionic Bonding
- Covalent Bonding
- Covalent bonding is the sharing of electrons between atoms. This bonding occurs primarily between nonmetals.
- IONIC BONDING
- This sort of bonding is important because it tends to hold differently charged atoms (i.e. metals and nonmetals), allowing for the many diverse types of objects we see around us. Ionic bonds also have a role in the dissolution of compounds in their polar solvents.
- I therefore conclude that
- Year 1896
- COVALENT BONDING
- Most carbon compounds interact predominantly by covalent bonding, hence covalent bonds are very significant. Covalent bonding allows molecules to share electrons with one another, allowing for the formation of vast chains of compounds and greater resistance in life.
- However, 
- Year 1896
- We are finally done! Thank you for helping me!
- Symonette Jazcel Q. Manalo11 Continental
- Year 1896
Sukurta daugiau nei 30 milijonų siužetinių lentelių

