atom boding
Siužetinės Linijos Tekstas
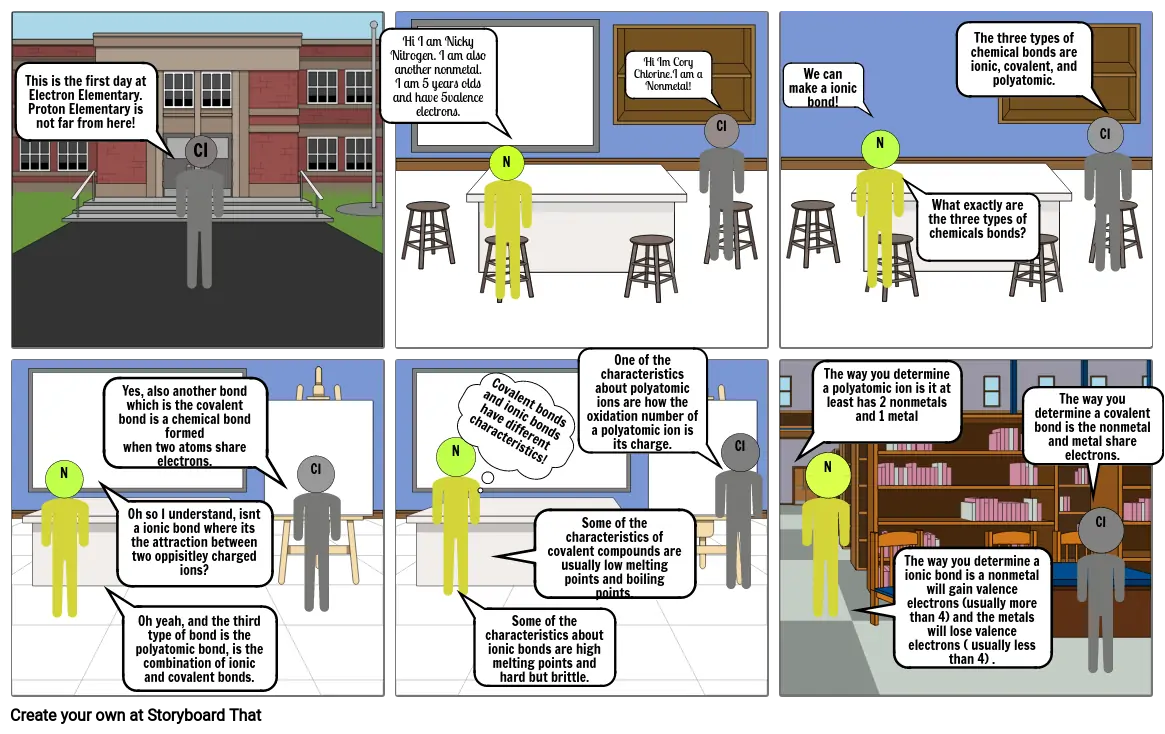
- This is the first day at Electron Elementary. Proton Elementary is not far from here!
- CI
- Hi I am Nicky Nitrogen. I am also another nonmetal.I am 5 years olds and have 5valence electrons.
- N
- One of the characteristics about polyatomic ions are how the oxidation number of a polyatomic ion is its charge.
- Hi Im Cory Chlorine.I am a Nonmetal!
- CI
- We can make a ionic bond!
- N
- What exactly are the three types of chemicals bonds?
- The three types of chemical bonds are ionic, covalent, and polyatomic.
- CI
- N
- Oh so I understand, isnt a ionic bond where its the attraction between two oppisitley charged ions?
- Yes, also another bond which is the covalent bond is a chemical bond formedwhen two atoms share electrons.
- Oh yeah, and the third type of bond is the polyatomic bond, is the combination of ionic and covalent bonds.
- CI
- N
- Covalent bonds and ionic bonds have different characteristics!
- Some of the characteristics about ionic bonds are high melting points and hard but brittle.
- Some of the characteristics of covalent compounds are usually low melting points and boiling points.
- CI
- The way you determine a polyatomic ion is it at least has 2 nonmetals and 1 metal
- N
- The way you determine a ionic bond is a nonmetal will gain valence electrons (usually more than 4) and the metals will lose valence electrons ( usually less than 4) .
- The way you determine a covalent bond is the nonmetal and metal share electrons.
- CI
Sukurta daugiau nei 30 milijonų siužetinių lentelių

