AGR 2
Testo Storyboard
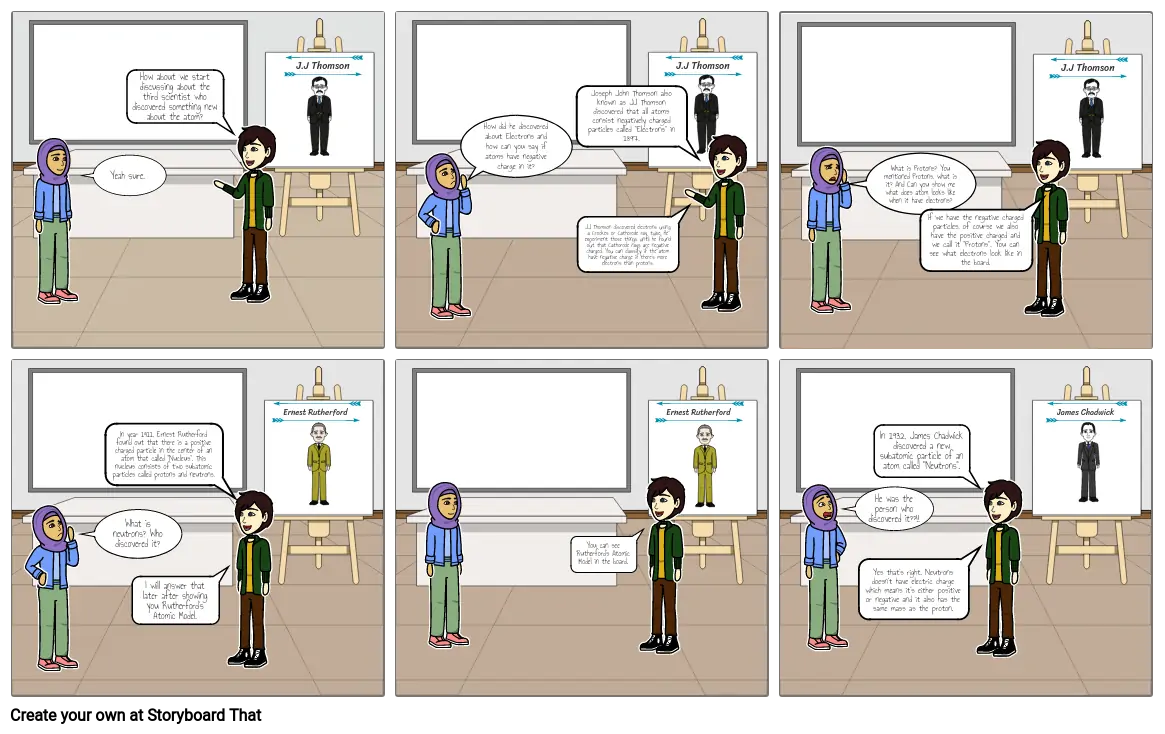
- Yeah sure.
- How about we start discussing about the third scientist who discovered something new about the atom?
- J.J Thomson
- How did he discovered about Electrons and how can you say if atoms have negative charge in it?
- Joseph John Thomson also known as JJ Thomson discovered that all atoms consist negatively charged particles called "Electrons" in 1897.
- JJ Thomson discovered electrons using a Crookes or Cathorode ray, tube. He experiment those things until he found out that Cathorode rays are negative charged. You can classify if the atom have negative charge if there's more electrons than protons.
- J.J Thomson
- What is Protons? You mentioned Protons, what is it? And Can you show me what does atom looks like when it have electrons?
- If we have the negative charged particles, of course we also have the positive charged and we call it "Protons". You can see what electrons look like in the board.
- J.J Thomson
- What is neutrons? Who discovered it?
- In year 1911, Ernest Rutherford found out that there is a positive charged particle in the center of an atom that called "Nucleus". This nucleus consists of two subatomic particles called protons and neutrons.
- I will answer that later after showing you Rutherford's Atomic Model.
- Ernest Rutherford
- You can see Rutherford's Atomic Model in the board.
- Ernest Rutherford
- He was the person who discovered it??!!
- Yes that's right. Neutrons doesn't have electric charge which means it's either positive or negative and it also has the same mass as the proton.
- In 1932, James Chadwick discovered a new subatomic particle of an atom called "Neutrons".
- James Chadwick
Oltre 30 milioni di storyboard creati

