History of atomic model
Testo Storyboard
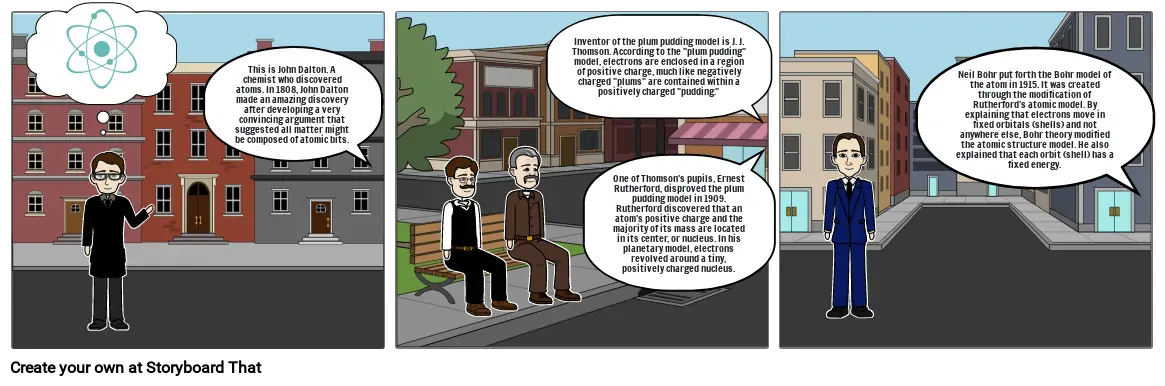
- This is John Dalton. A chemist who discovered atoms. In 1808, John Dalton made an amazing discovery after developing a very convincing argument that suggested all matter might be composed of atomic bits.
- Inventor of the plum pudding model is J. J. Thomson. According to the plum pudding model, electrons are enclosed in a region of positive charge, much like negatively charged plums are contained within a positively charged pudding.
- One of Thomson's pupils, Ernest Rutherford, disproved the plum pudding model in 1909. Rutherford discovered that an atom's positive charge and the majority of its mass are located in its center, or nucleus. In his planetary model, electrons revolved around a tiny, positively charged nucleus.
- Neil Bohr put forth the Bohr model of the atom in 1915. It was created through the modification of Rutherford's atomic model. By explaining that electrons move in fixed orbitals (shells) and not anywhere else, Bohr theory modified the atomic structure model. He also explained that each orbit (shell) has a fixed energy.
Oltre 30 milioni di storyboard creati

