Chemistry CAP
Testo Storyboard
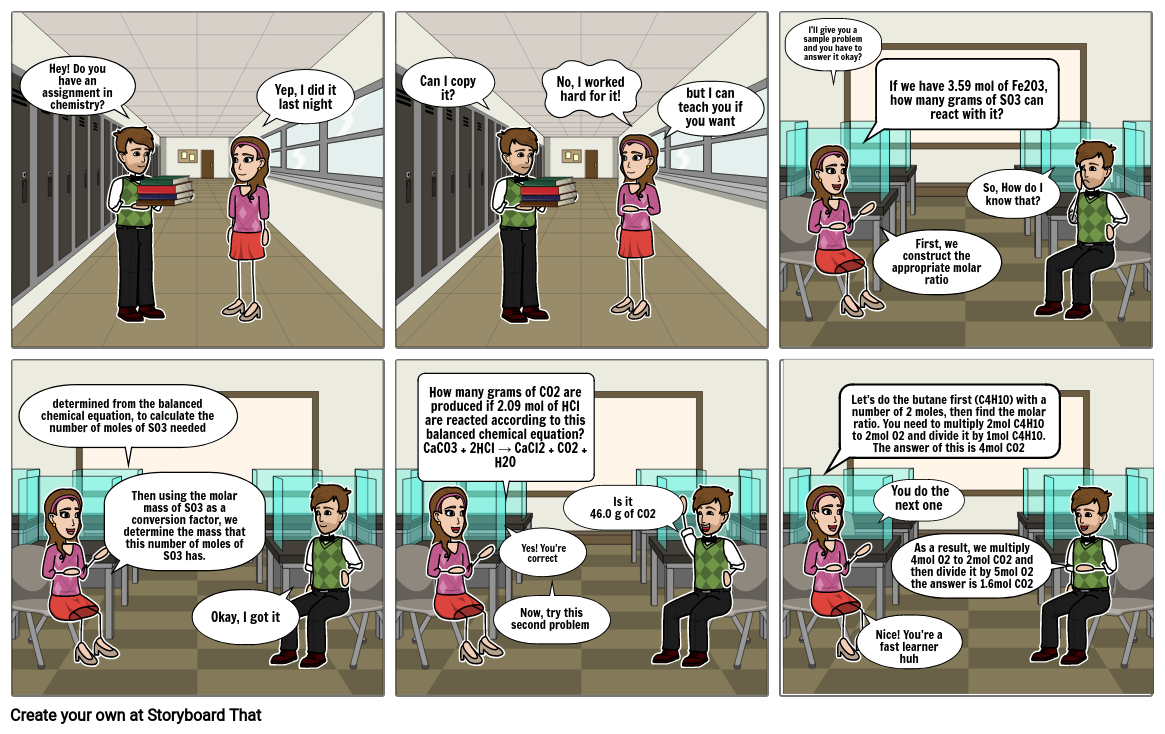
- Hey! Do you have an assignment in chemistry?
- Yep, I did it last night
- Can I copy it?
- No, I worked hard for it!
- but I can teach you if you want
- I’ll give you a sample problem and you have to answer it okay?
- If we have 3.59 mol of Fe2O3, how many grams of SO3 can react with it?
- First, we construct the appropriate molar ratio
- So, How do I know that?
- determined from the balanced chemical equation, to calculate the number of moles of SO3 needed
- Then using the molar mass of SO3 as a conversion factor, we determine the mass that this number of moles of SO3 has.
- Okay, I got it
- How many grams of CO2 are produced if 2.09 mol of HCl are reacted according to this balanced chemical equation?CaCO3 + 2HCI → CaCI2 + CO2 + H2O
- Now, try this second problem
- Yes! You’re correct
- Is it46.0 g of CO2
- Let’s do the butane first (C4H10) with a number of 2 moles, then find the molar ratio. You need to multiply 2mol C4H10 to 2mol O2 and divide it by 1mol C4H10. The answer of this is 4mol CO2
- Nice! You’re a fast learner huh
- You do the next one
- As a result, we multiply 4mol O2 to 2mol CO2 and then divide it by 5mol O2 the answer is 1.6mol CO2
Oltre 30 milioni di storyboard creati

