Chemistry SEA project
Storyboard Szöveg
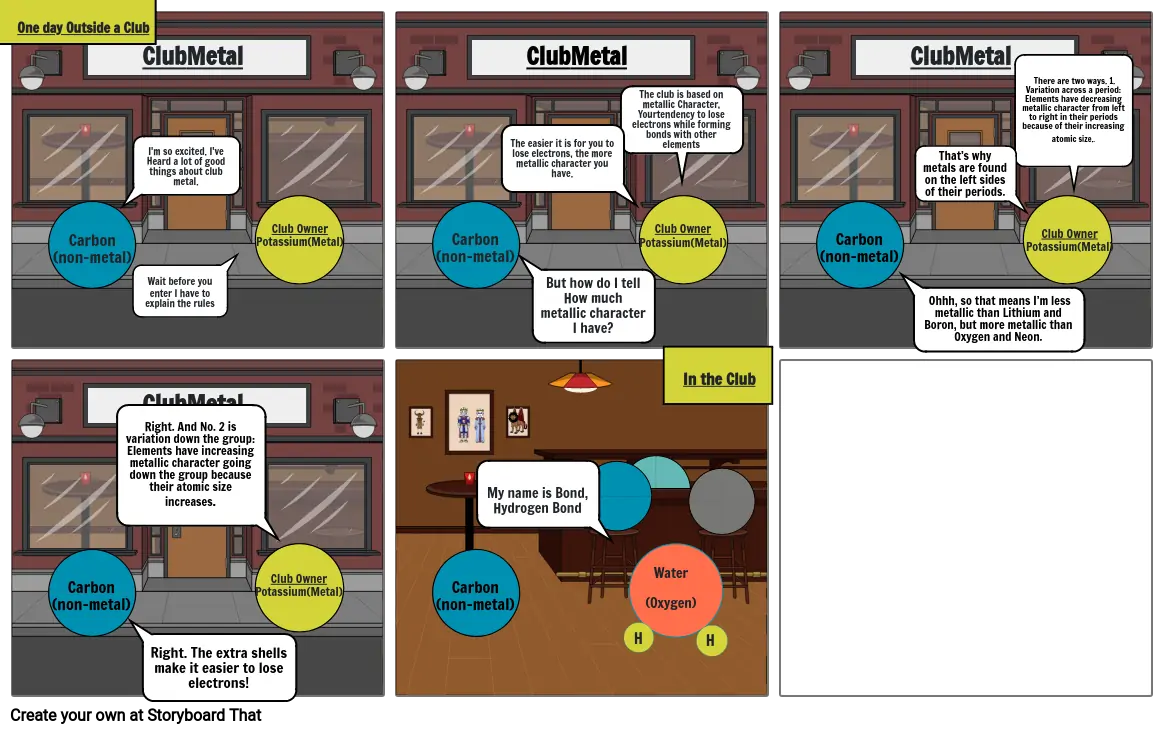
- One day Outside a Club
- Carbon(non-metal)
- ClubMetal
- ClubMetal
- I'm so excited. I've Heard a lot of good things about club metal.
- Wait before you enter I have to explain the rules
- Club OwnerPotassium(Metal)
- Carbon(non-metal)
- ClubMetal
- The easier it is for you to lose electrons, the more metallic character you have.
- But how do I tell How much metallic character I have?
- The club is based on metallic Character, Yourtendency to lose electrons while forming bonds with other elements
- Club OwnerPotassium(Metal)
- In the Club
- Carbon(non-metal)
- ClubMetal
- Ohhh, so that means I’m less metallic than Lithium and Boron, but more metallic than Oxygen and Neon.
- That’s why metals are found on the left sides of their periods.
- There are two ways. 1. Variation across a period: Elements have decreasing metallic character from left to right in their periods because of their increasing atomic size..
- Club OwnerPotassium(Metal)
- Carbon(non-metal)
- Right. And No. 2 is variation down the group: Elements have increasing metallic character going down the group because their atomic size increases.
- Right. The extra shells make it easier to lose electrons!
- Club OwnerPotassium(Metal)
- Carbon(non-metal)
- My name is Bond,Hydrogen Bond
- Water(Oxygen)
- H
- H
Több mint 30 millió storyboard készült

