Storyboard Szöveg
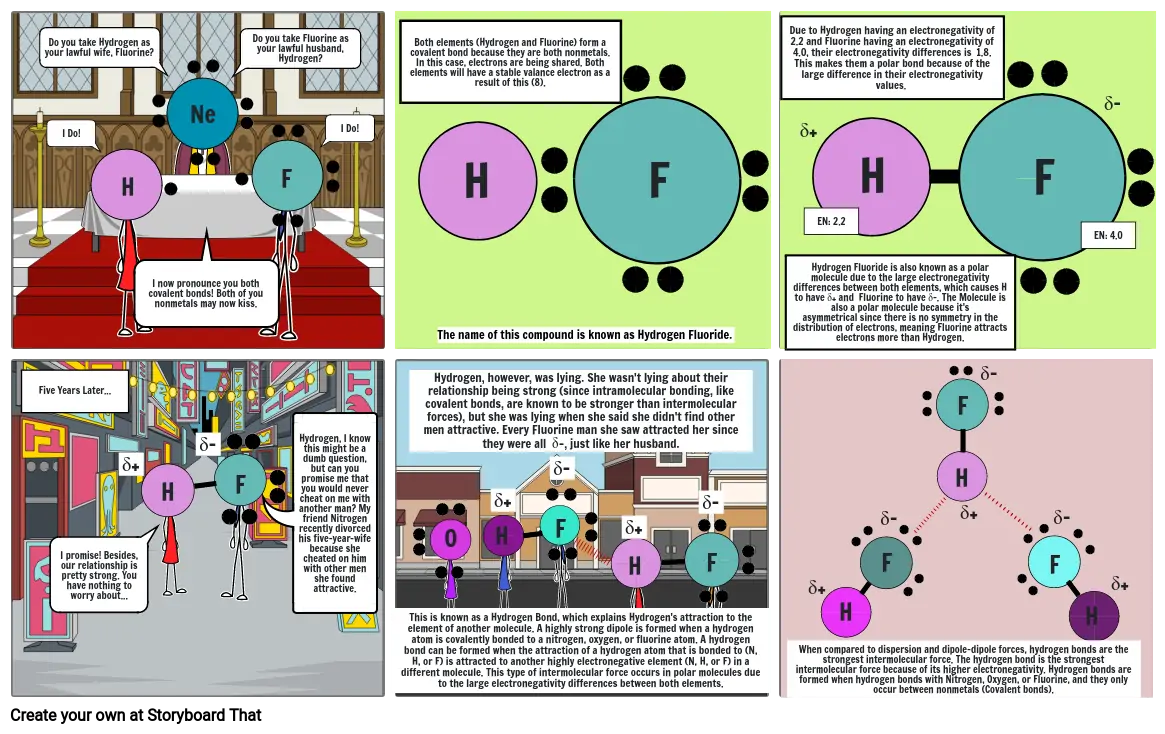
- Do you take Hydrogen as your lawful wife, Fluorine?
- I Do!
- H
- I now pronounce you both covalent bonds! Both of you nonmetals may now kiss.
-
-
-
-
- Ne
-
-
-
-
-
-
- F
-
-
-
-
- I Do!
-
-
- Do you take Fluorine as your lawful husband, Hydrogen?
- Both elements (Hydrogen and Fluorine) form a covalent bond because they are both nonmetals. In this case, electrons are being shared. Both elements will have a stable valance electron as a result of this (8).
- The name of this compound is known as Hydrogen Fluoride.
- H
-
-
- F
-
-
-
-
-
-
- Due to Hydrogen having an electronegativity of 2.2 and Fluorine having an electronegativity of 4.0, their electronegativity differences is 1.8. This makes them a polar bond because of the large difference in their electronegativity values.
- Hydrogen Fluoride is also known as a polar molecule due to the large electronegativity differences between both elements, which causes H to have δ+ and Fluorine to have δ-. The Molecule is also a polar molecule because it's asymmetrical since there is no symmetry in the distribution of electrons, meaning Fluorine attracts electrons more than Hydrogen.
- δ+
- EN: 2.2
- H
- F
-
-
-
-
- EN: 4.0
- δ-
-
-
- Five Years Later...
- δ+
- H
- I promise! Besides, our relationship is pretty strong. You have nothing to worry about...
- δ-
-
- F
-
-
-
- Hydrogen, I know this might be a dumb question, but can you promise me that you would never cheat on me with another man? My friend Nitrogen recently divorced his five-year-wife because she cheated on him with other men she found attractive.
-
-
- This is known as a Hydrogen Bond, which explains Hydrogen's attraction to the element of another molecule. A highly strong dipole is formed when a hydrogen atom is covalently bonded to a nitrogen, oxygen, or fluorine atom. A hydrogen bond can be formed when the attraction of a hydrogen atom that is bonded to (N, H, or F) is attracted to another highly electronegative element (N, H, or F) in a different molecule. This type of intermolecular force occurs in polar molecules due to the large electronegativity differences between both elements.
- Hydrogen, however, was lying. She wasn't lying about their relationship being strong (since intramolecular bonding, like covalent bonds, are known to be stronger than intermolecular forces), but she was lying when she said she didn't find other men attractive. Every Fluorine man she saw attracted her since they were all δ-, just like her husband.
-
-
- O
-
-
-
-
- H
- δ+
- F
-
-
- δ-
-
-
-
-
- H
- δ+
- F
-
- δ-
-
-
-
-
-
- When compared to dispersion and dipole-dipole forces, hydrogen bonds are the strongest intermolecular force. The hydrogen bond is the strongest intermolecular force because of its higher electronegativity. Hydrogen bonds are formed when hydrogen bonds with Nitrogen, Oxygen, or Fluorine, and they only occur between nonmetals (Covalent bonds).
- δ+
-
- H
-
-
- F
- δ-
-
-
-
-
-
-
- H
- F
-
- δ+
-
- δ-
-
-
-
-
-
- F
-
- δ-
-
- H
-
- δ+
Több mint 30 millió storyboard készült

