Experiment 9.2: Forming a Precipitate
Storyboard Szöveg
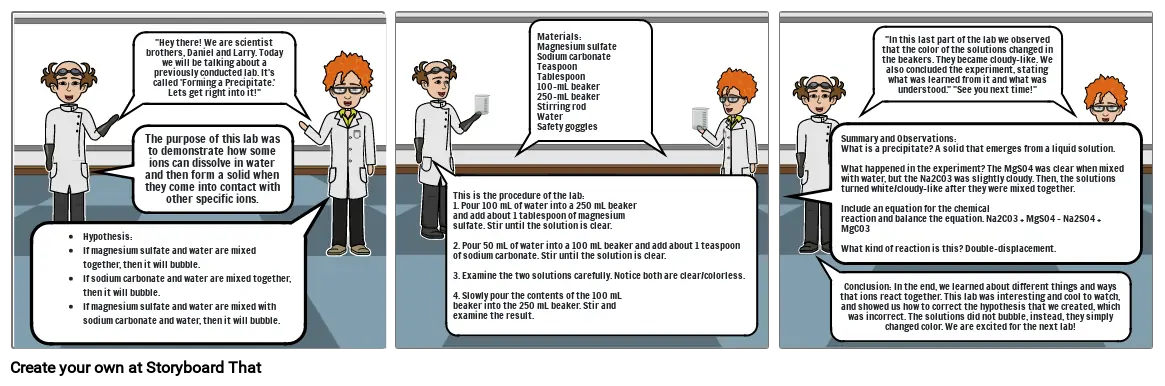
- Hypothesis:If magnesium sulfate and water are mixed together, then it will bubble.If sodium carbonate and water are mixed together, then it will bubble.If magnesium sulfate and water are mixed with sodium carbonate and water, then it will bubble.
- The purpose of this lab was to demonstrate how some ions can dissolve in water and then form a solid when they come into contact with other specific ions.
- "Hey there! We are scientist brothers, Daniel and Larry. Today we will be talking about a previously conducted lab. It's called 'Forming a Precipitate.'Lets get right into it!"
- This is the procedure of the lab:1. Pour 100 mL of water into a 250 mL beakerand add about 1 tablespoon of magnesiumsulfate. Stir until the solution is clear.2. Pour 50 mL of water into a 100 mL beaker and add about 1 teaspoon of sodium carbonate. Stir until the solution is clear.3. Examine the two solutions carefully. Notice both are clear/colorless. 4. Slowly pour the contents of the 100 mLbeaker into the 250 mL beaker. Stir andexamine the result.
- Materials:Magnesium sulfateSodium carbonateTeaspoonTablespoon100-mL beaker250-mL beakerStirring rodWaterSafety goggles
- Summary and Observations:What is a precipitate? A solid that emerges from a liquid solution.What happened in the experiment? The MgSO4 was clear when mixed with water, but the Na2CO3 was slightly cloudy. Then, the solutions turned white/cloudy-like after they were mixed together. Include an equation for the chemicalreaction and balance the equation. Na2CO3 + MgSO4 - Na2SO4 + MgCO3What kind of reaction is this? Double-displacement.
- Conclusion: In the end, we learned about different things and ways that ions react together. This lab was interesting and cool to watch, and showed us how to correct the hypothesis that we created, which was incorrect. The solutions did not bubble, instead, they simply changed color. We are excited for the next lab!
- "In this last part of the lab we observed that the color of the solutions changed in the beakers. They became cloudy-like. We also concluded the experiment, stating what was learned from it and what was understood." "See you next time!"
Több mint 30 millió storyboard készült

