Intermolecular Forces Part 2
स्टोरीबोर्ड पाठ
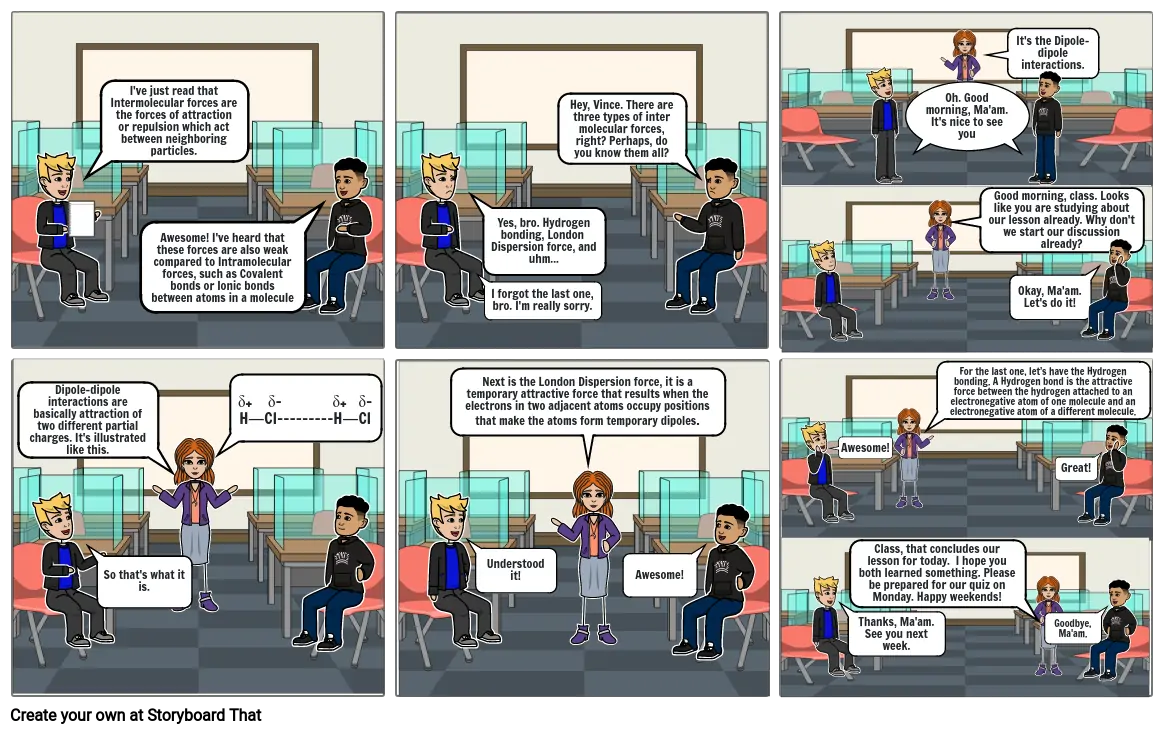
- I've just read that Intermolecular forces are the forces of attraction or repulsion which act between neighboring particles.
- Awesome! I've heard that these forces are also weak compared to Intramolecular forces, such as Covalent bonds or Ionic bonds between atoms in a molecule
- Yes, bro. Hydrogen bonding, London Dispersion force, and uhm...
- I forgot the last one, bro. I'm really sorry.
- Hey, Vince. There are three types of inter molecular forces, right? Perhaps, do you know them all?
- Oh. Good morning, Ma'am. It's nice to see you
- Good morning, class. Looks like you are studying about our lesson already. Why don't we start our discussion already?
- It's the Dipole-dipole interactions.
- Okay, Ma'am. Let's do it!
- Dipole-dipole interactions are basically attraction of two different partial charges. It's illustrated like this.
- So that's what it is.
- δ+ δ- δ+ δ-H―Cl---------H―Cl
- Next is the London Dispersion force, it is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles.
- Understood it!
- Awesome!
- Awesome!
- Thanks, Ma'am. See you next week.
- Class, that concludes our lesson for today. I hope you both learned something. Please be prepared for our quiz on Monday. Happy weekends!
- For the last one, let’s have the Hydrogen bonding. A Hydrogen bond is the attractive force between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of a different molecule.
- Goodbye, Ma'am.
- Great!
30 मिलियन से अधिक स्टोरीबोर्ड बनाए गए

