Science project
स्टोरीबोर्ड पाठ
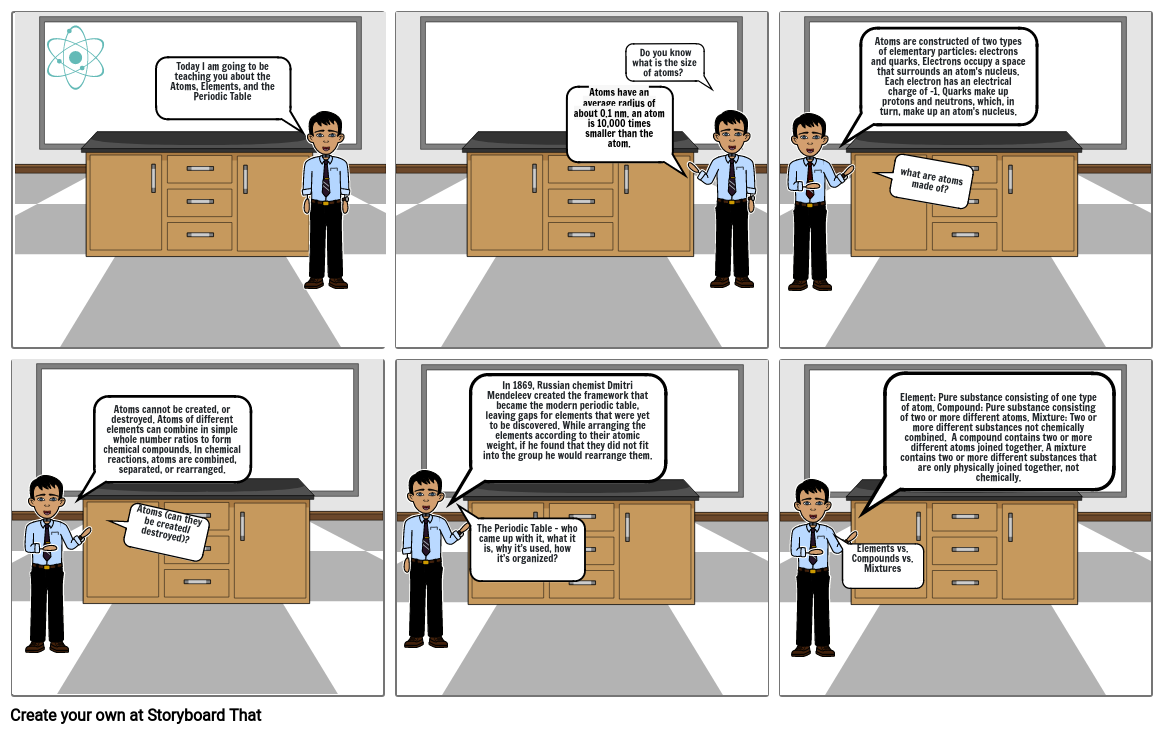
- Today I am going to be teaching you about the Atoms, Elements, and the Periodic Table
- Atoms have an average radius of about 0.1 nm. an atom is 10,000 times smaller than the atom.
- Do you know what is the size of atoms?
- Atoms are constructed of two types of elementary particles: electrons and quarks. Electrons occupy a space that surrounds an atom's nucleus. Each electron has an electrical charge of -1. Quarks make up protons and neutrons, which, in turn, make up an atom's nucleus.
- what are atoms made of?
- Atoms cannot be created, or destroyed. Atoms of different elements can combine in simple whole number ratios to form chemical compounds. In chemical reactions, atoms are combined, separated, or rearranged.
- Atoms (can they be created/ destroyed)?
- In 1869, Russian chemist Dmitri Mendeleev created the framework that became the modern periodic table, leaving gaps for elements that were yet to be discovered. While arranging the elements according to their atomic weight, if he found that they did not fit into the group he would rearrange them.
- The Periodic Table - who came up with it, what it is, why it’s used, how it’s organized?
- Elements vs. Compounds vs. Mixtures
- Element: Pure substance consisting of one type of atom. Compound: Pure substance consisting of two or more different atoms. Mixture: Two or more different substances not chemically combined. A compound contains two or more different atoms joined together. A mixture contains two or more different substances that are only physically joined together, not chemically.
30 मिलियन से अधिक स्टोरीबोर्ड बनाए गए

