covalent
स्टोरीबोर्ड पाठ
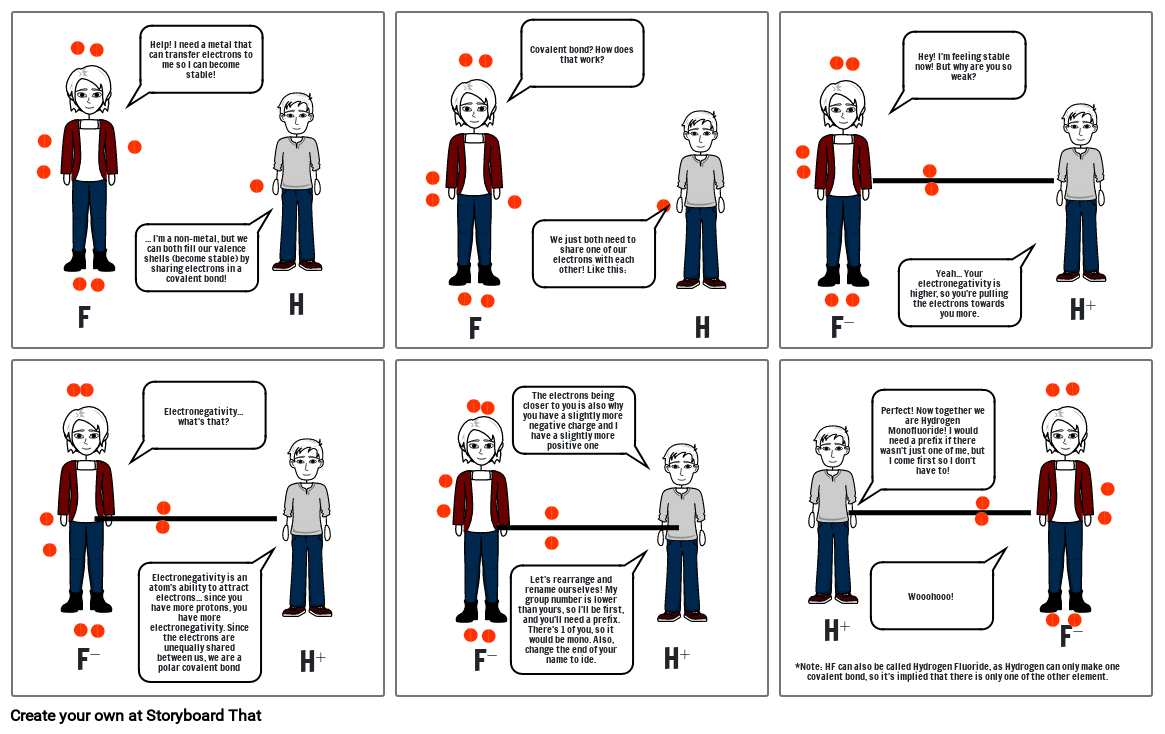
- F
- Help! I need a metal that can transfer electrons to me so I can become stable!
- ... I'm a non-metal, but we can both fill our valence shells (become stable) by sharing electrons in a covalent bond!
- H
- F
- Covalent bond? How does that work?
- We just both need to share one of our electrons with each other! Like this:
- H
- F⁻
- Hey! I'm feeling stable now! But why are you so weak?
- Yeah... Your electronegativity is higher, so you're pulling the electrons towards you more.
- H⁺
- F⁻
- Electronegativity... what's that?
- Electronegativity is an atom's ability to attract electrons... since you have more protons, you have more electronegativity. Since the electrons are unequally shared between us, we are a polar covalent bond
- H⁺
- F⁻
- Let's rearrange and rename ourselves! My group number is lower than yours, so I'll be first, and you'll need a prefix. There's 1 of you, so it would be mono. Also, change the end of your name to ide.
- The electrons being closer to you is also why you have a slightly more negative charge and I have a slightly more positive one
- H⁺
- H⁺
- *Note: HF can also be called Hydrogen Fluoride, as Hydrogen can only make one covalent bond, so it's implied that there is only one of the other element.
- Perfect! Now together we are Hydrogen Monofluoride! I would need a prefix if there wasn't just one of me, but I come first so I don't have to!
- Wooohooo!
- F⁻
30 मिलियन से अधिक स्टोरीबोर्ड बनाए गए
कोई डाउनलोड नहीं, कोई क्रेडिट कार्ड नहीं, और कोशिश करने के लिए किसी लॉगिन की आवश्यकता नहीं है!
