Chemical Comic Strip
Texte du Storyboard
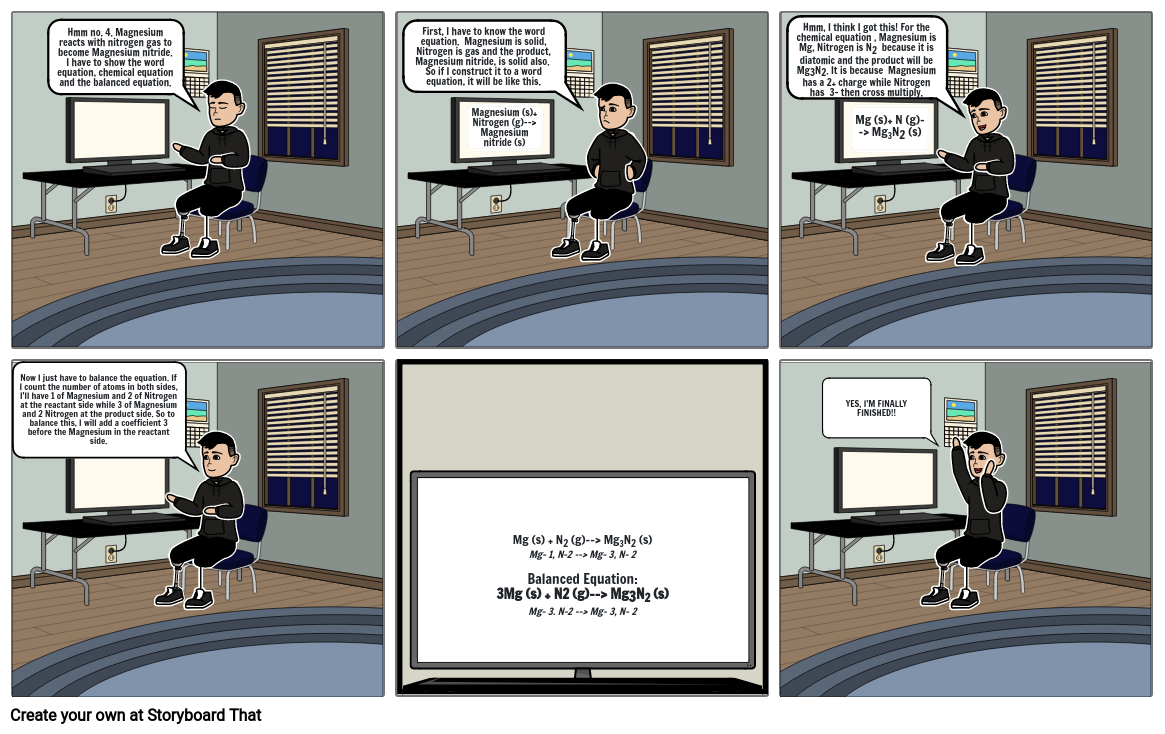
- Hmm no. 4. Magnesium reacts with nitrogen gas to become Magnesium nitride. I have to show the word equation, chemical equation and the balanced equation.
- First, I have to know the word equation. Magnesium is solid, Nitrogen is gas and the product, Magnesium nitride, is solid also. So if I construct it to a word equation, it will be like this.
- Magnesium (s)+ Nitrogen (g)--> Magnesium nitride (s)
- Hmm, I think I got this! For the chemical equation , Magnesium is Mg, Nitrogen is N2 because it is diatomic and the product will be Mg3N2. It is because Magnesium has a 2+ charge while Nitrogen has 3- then cross multiply.
- Mg (s)+ N (g)--> Mg3N2 (s)
- Now I just have to balance the equation. If I count the number of atoms in both sides, I'll have 1 of Magnesium and 2 of Nitrogen at the reactant side while 3 of Magnesium and 2 Nitrogen at the product side. So to balance this, I will add a coefficient 3 before the Magnesium in the reactant side.
- Mg (s) + N2 (g)--> Mg3N2 (s)Mg- 1, N-2 --> Mg- 3, N- 2Balanced Equation:3Mg (s) + N2 (g)--> Mg3N2 (s)Mg- 3. N-2 --> Mg- 3, N- 2
- YES, I'M FINALLY FINISHED!!
Plus de 30 millions de storyboards créés

