Unknown Story
Texte du Storyboard
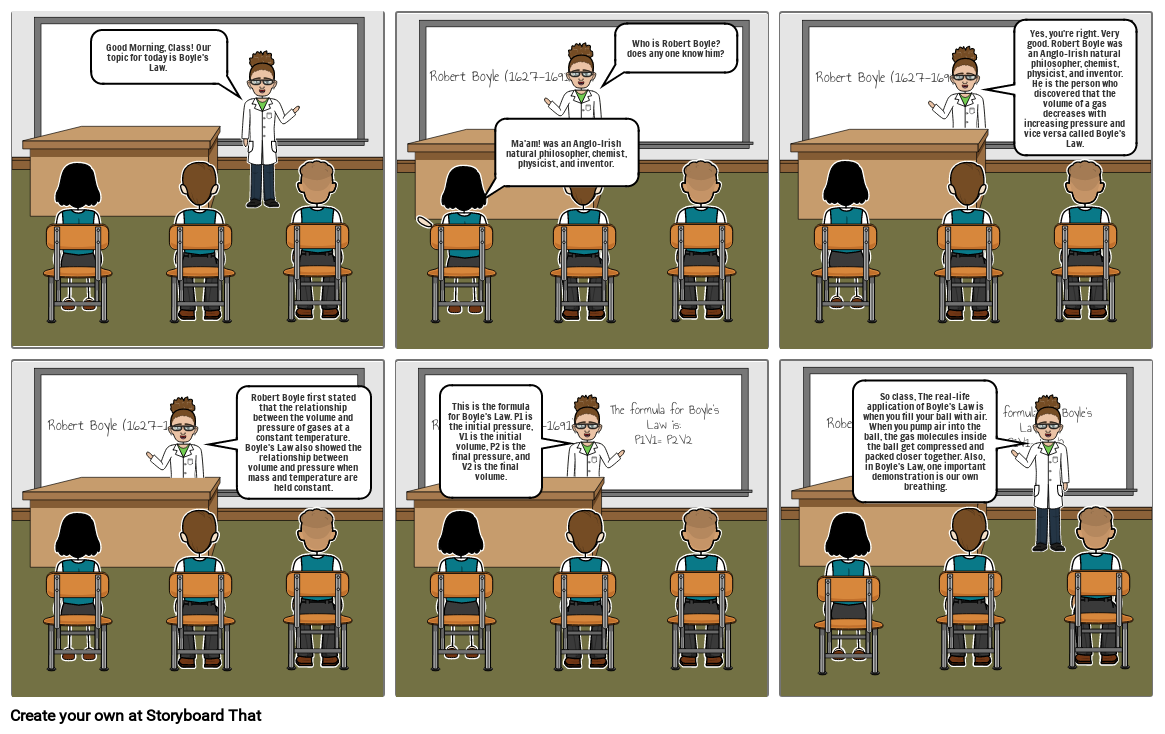
- Good Morning, Class! Our topic for today is Boyle's Law.
- Robert Boyle (1627-1691)
- Ma'am! was an Anglo-Irish natural philosopher, chemist, physicist, and inventor.
- Who is Robert Boyle? does any one know him?
- Robert Boyle (1627-1691)
- Yes, you're right. Very good. Robert Boyle was an Anglo-Irish natural philosopher, chemist, physicist, and inventor. He is the person who discovered that the volume of a gas decreases with increasing pressure and vice versa called Boyle's Law.
- Robert Boyle (1627-1691)
- Robert Boyle first stated that the relationship between the volume and pressure of gases at a constant temperature. Boyle's Law also showed the relationship between volume and pressure when mass and temperature are held constant.
- Robert Boyle (1627-1691)
- This is the formula for Boyle's Law. P1 is the initial pressure, V1 is the initial volume, P2 is the final pressure, and V2 is the final volume.
- The formula for Boyle’s Law is: P1V1= P2V2
- Robert Boyle (1627-1691)
- The formula for Boyle’s Law is: P1V1= P2V2
- So class, The real-life application of Boyle's Law is when you fill your ball with air. When you pump air into the ball, the gas molecules inside the ball get compressed and packed closer together. Also, in Boyle's Law, one important demonstration is our own breathing.
Plus de 30 millions de storyboards créés

