Kuvakäsikirjoitus Teksti
- Me too! I can't wait for us to float above the clouds!!
- I'm happy and excited! It's my first time riding in a hot air balloon!
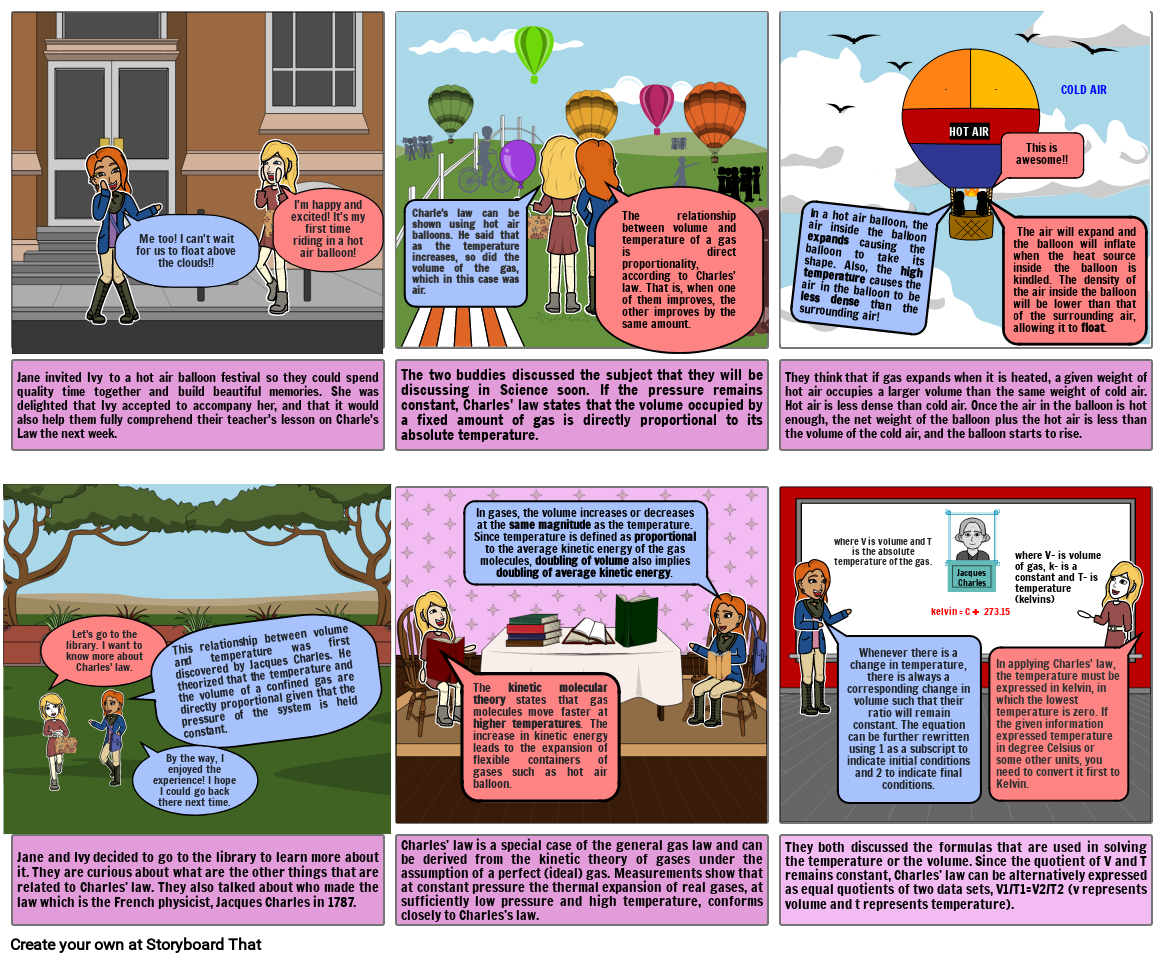
- Charle's law can be shown using hot air balloons. He said that as the temperature increases, so did the volume of the gas, which in this case was air.
-
- In a hot air balloon, the air inside the balloon expands causing the balloon to take its shape. Also, the high temperature causes the air in the balloon to be less dense than the surrounding air!
- . .
- HOT AIR
- This is awesome!!
- COLD AIR
- The air will expand and the balloon will inflate when the heat source inside the balloon is kindled. The density of the air inside the balloon will be lower than that of the surrounding air, allowing it to float.
- Jane invited Ivy to a hot air balloon festival so they could spend quality time together and build beautiful memories. She was delighted that Ivy accepted to accompany her, and that it would also help them fully comprehend their teacher's lesson on Charle's Law the next week.
- Let's go to the library. I want to know more about Charles' law.
- This relationship between volume and temperature was first discovered by Jacques Charles. He theorized that the temperature and the volume of a confined gas are directly proportional given that the pressure of the system is held constant.
- The two buddies discussed the subject that they will be discussing in Science soon. If the pressure remains constant, Charles' law states that the volume occupied by a fixed amount of gas is directly proportional to its absolute temperature.
- The kinetic molecular theory states that gas molecules move faster at higher temperatures. The increase in kinetic energy leads to the expansion of flexible containers of gases such as hot air balloon.
- In gases, the volume increases or decreases at the same magnitude as the temperature. Since temperature is defined as proportional to the average kinetic energy of the gas molecules, doubling of volume also implies doubling of average kinetic energy.
- The relationship between volume and temperature of a gas is direct proportionality, according to Charles' law. That is, when one of them improves, the other improves by the same amount.
- They think that if gas expands when it is heated, a given weight of hot air occupies a larger volume than the same weight of cold air. Hot air is less dense than cold air. Once the air in the balloon is hot enough, the net weight of the balloon plus the hot air is less than the volume of the cold air, and the balloon starts to rise.
- Whenever there is a change in temperature, there is always a corresponding change in volume such that their ratio will remain constant. The equation can be further rewritten using 1 as a subscript to indicate initial conditions and 2 to indicate final conditions.
- where V is volume and T is the absolute temperature of the gas.
- kelvin = C + 273.15
-
- Jacques Charles
- In applying Charles' law, the temperature must be expressed in kelvin, in which the lowest temperature is zero. If the given information expressed temperature in degree Celsius or some other units, you need to convert it first to Kelvin.
- where V- is volume of gas, k- is a constant and T- is temperature (kelvins)
- Jane and Ivy decided to go to the library to learn more about it. They are curious about what are the other things that are related to Charles' law. They also talked about who made the law which is the French physicist, Jacques Charles in 1787.
- By the way, I enjoyed the experience! I hope I could go back there next time.
- Charles' law is a special case of the general gas law and can be derived from the kinetic theory of gases under the assumption of a perfect (ideal) gas. Measurements show that at constant pressure the thermal expansion of real gases, at sufficiently low pressure and high temperature, conforms closely to Charles’s law.
- They both discussed the formulas that are used in solving the temperature or the volume. Since the quotient of V and T remains constant, Charles’ law can be alternatively expressed as equal quotients of two data sets, V1/T1=V2/T2 (v represents volume and t represents temperature).
Yli 30 miljoonaa kuvakäsikirjoitusta luotu

