Rutherford vs Thompson comic
Kuvakäsikirjoitus Teksti
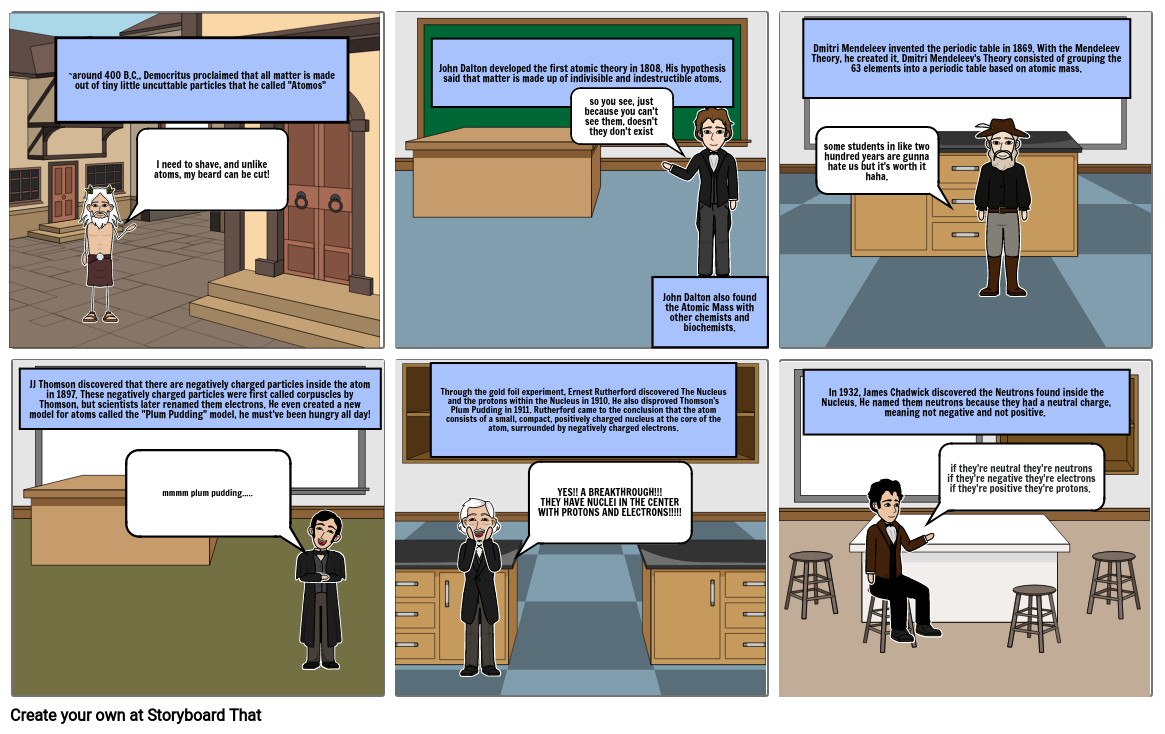
- ~around 400 B.C., Democritus proclaimed that all matter is made out of tiny little uncuttable particles that he called "Atomos"
- I need to shave, and unlike atoms, my beard can be cut!
- John Dalton developed the first atomic theory in 1808. His hypothesis said that matter is made up of indivisible and indestructible atoms.
- so you see, just because you can't see them, doesn't they don't exist
- John Dalton also found the Atomic Mass with other chemists and biochemists.
- Dmitri Mendeleev invented the periodic table in 1869. With the Mendeleev Theory, he created it. Dmitri Mendeleev's Theory consisted of grouping the 63 elements into a periodic table based on atomic mass.
- some students in like two hundred years are gunna hate us but it's worth it haha.
- JJ Thomson discovered that there are negatively charged particles inside the atom in 1897. These negatively charged particles were first called corpuscles by Thomson, but scientists later renamed them electrons. He even created a new model for atoms called the "Plum Pudding" model, he must've been hungry all day!
- mmmm plum pudding.....
- Through the gold foil experiment, Ernest Rutherford discovered The Nucleus and the protons within the Nucleus in 1910. He also disproved Thomson's Plum Pudding in 1911. Rutherford came to the conclusion that the atom consists of a small, compact, positively charged nucleus at the core of the atom, surrounded by negatively charged electrons.
- YES!! A BREAKTHROUGH!!!THEY HAVE NUCLEI IN THE CENTER WITH PROTONS AND ELECTRONS!!!!!
- In 1932, James Chadwick discovered the Neutrons found inside the Nucleus. He named them neutrons because they had a neutral charge, meaning not negative and not positive.
- if they're neutral they're neutronsif they're negative they're electronsif they're positive they're protons.
Yli 30 miljoonaa kuvakäsikirjoitusta luotu

