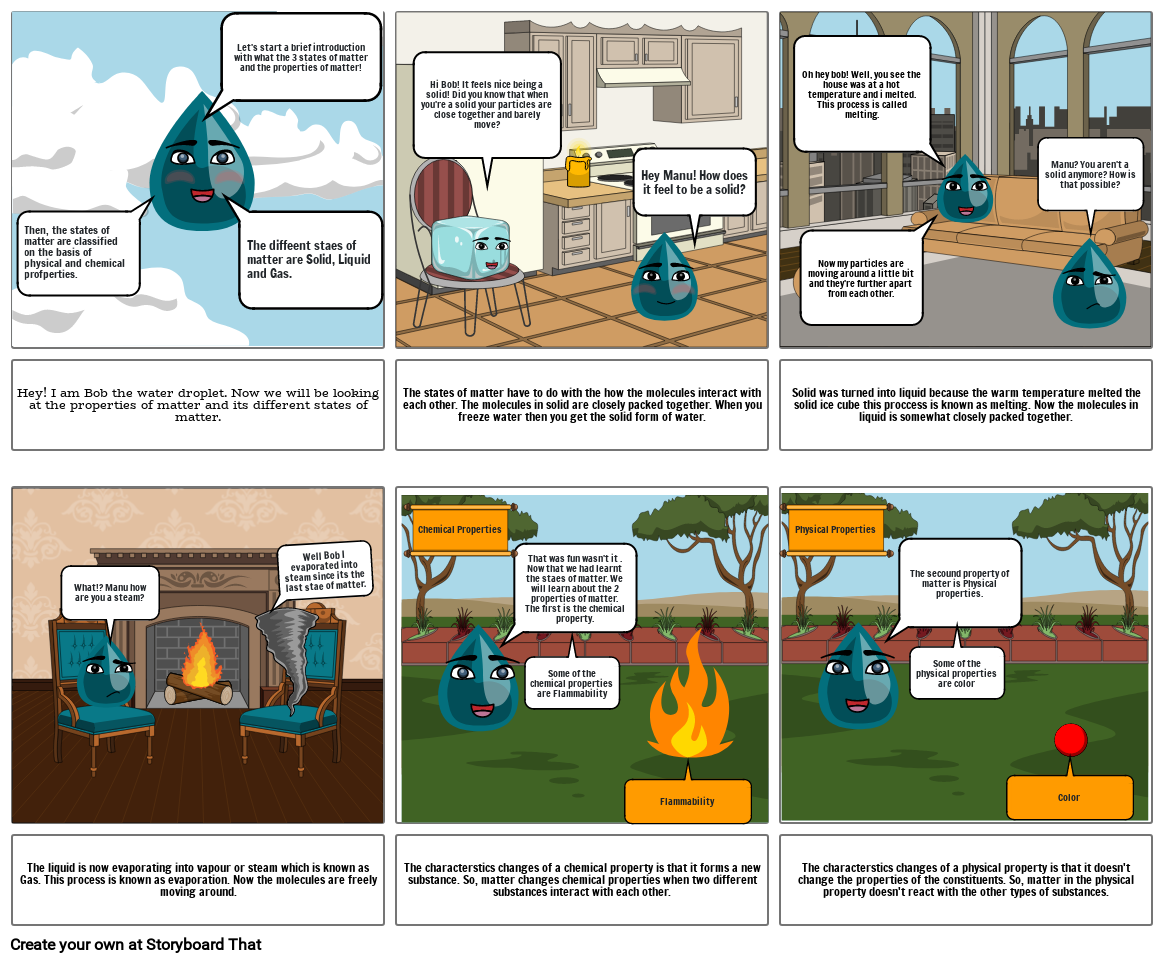
Properties of Particles in Matter.
Kuvakäsikirjoitus Teksti
- Then, the states of matter are classified on the basis of physical and chemical profperties.
- Let's start a brief introduction with what the 3 states of matter and the properties of matter!
- The diffeent staes of matter are Solid, Liquid and Gas.
- Hi Bob! It feels nice being a solid! Did you know that when you're a solid your particles are close together and barely move?
- Hey Manu! How does it feel to be a solid?
- Oh hey bob! Well, you see the house was at a hot temperature and i melted. This process is called melting.
- Now my particles are moving around a little bit and they're further apart from each other.
- Manu? You aren't a solid anymore? How is that possible?
- Hey! I am Bob the water droplet. Now we will be looking at the properties of matter and its different states of matter.
- What!? Manu how are you a steam?
- Well Bob I evaporated into steam since its the last stae of matter.
- The states of matter have to do with the how the molecules interact with each other. The molecules in solid are closely packed together. When you freeze water then you get the solid form of water.
- Chemical Properties
- That was fun wasn't it . Now that we had learnt the staes of matter. We will learn about the 2 properties of matter.The first is the chemical property.
- Physical Properties
- Solid was turned into liquid because the warm temperature melted the solid ice cube this proccess is known as melting. Now the molecules in liquid is somewhat closely packed together.
- The secound property of matter is Physical properties.
- The liquid is now evaporating into vapour or steam which is known as Gas. This process is known as evaporation. Now the molecules are freely moving around.
- The characterstics changes of a chemical property is that it forms a new substance. So, matter changes chemical properties when two different substances interact with each other.
- Some of the chemical properties are Flammability
- Flammability
- The characterstics changes of a physical property is that it doesn't change the properties of the constituents. So, matter in the physical property doesn't react with the other types of substances.
- Some of the physical properties are color
- Color
Yli 30 miljoonaa kuvakäsikirjoitusta luotu

