Chemistry
Süžeeskeem Tekst
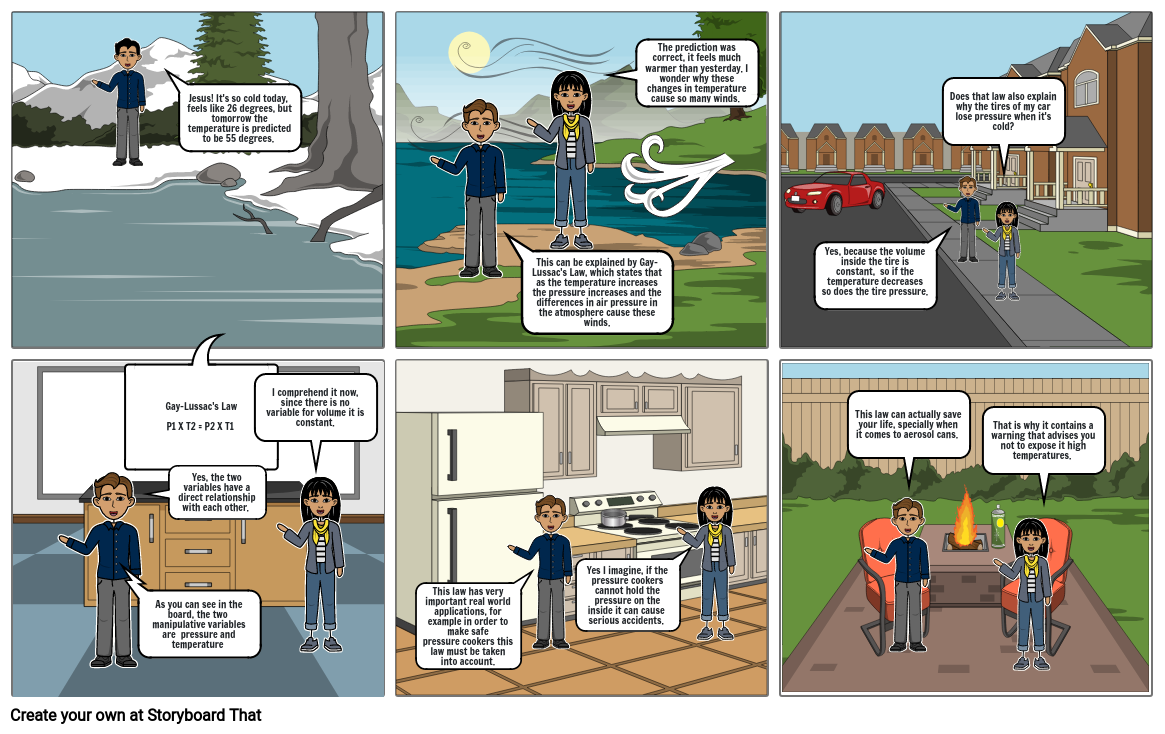
- Gay-Lussac's LawP1 X T2 = P2 X T1
- Jesus! It's so cold today, feels like 26 degrees, but tomorrow the temperature is predicted to be 55 degrees.
- This can be explained by Gay-Lussac's Law, which states that as the temperature increases the pressure increases and the differences in air pressure in the atmosphere cause these winds.
- The prediction was correct, it feels much warmer than yesterday. I wonder why these changes in temperature cause so many winds.
- Yes, because the volume inside the tire is constant, so if the temperature decreases so does the tire pressure.
- Does that law also explain why the tires of my car lose pressure when it's cold?
- As you can see in the board, the two manipulative variables are pressure and temperature
- Yes, the two variables have a direct relationship with each other.
- I comprehend it now, since there is no variable for volume it is constant.
- This law has very important real world applications, for example in order to make safe pressure cookers this law must be taken into account.
- Yes I imagine, if the pressure cookers cannot hold the pressure on the inside it can cause serious accidents.
- This law can actually save your life, specially when it comes to aerosol cans.
- That is why it contains a warning that advises you not to expose it high temperatures.
Loodud üle 30 miljoni süžeeskeemi

