Use the kinetic molecular model to explain properties of states of matter
Texto del Guión Gráfico
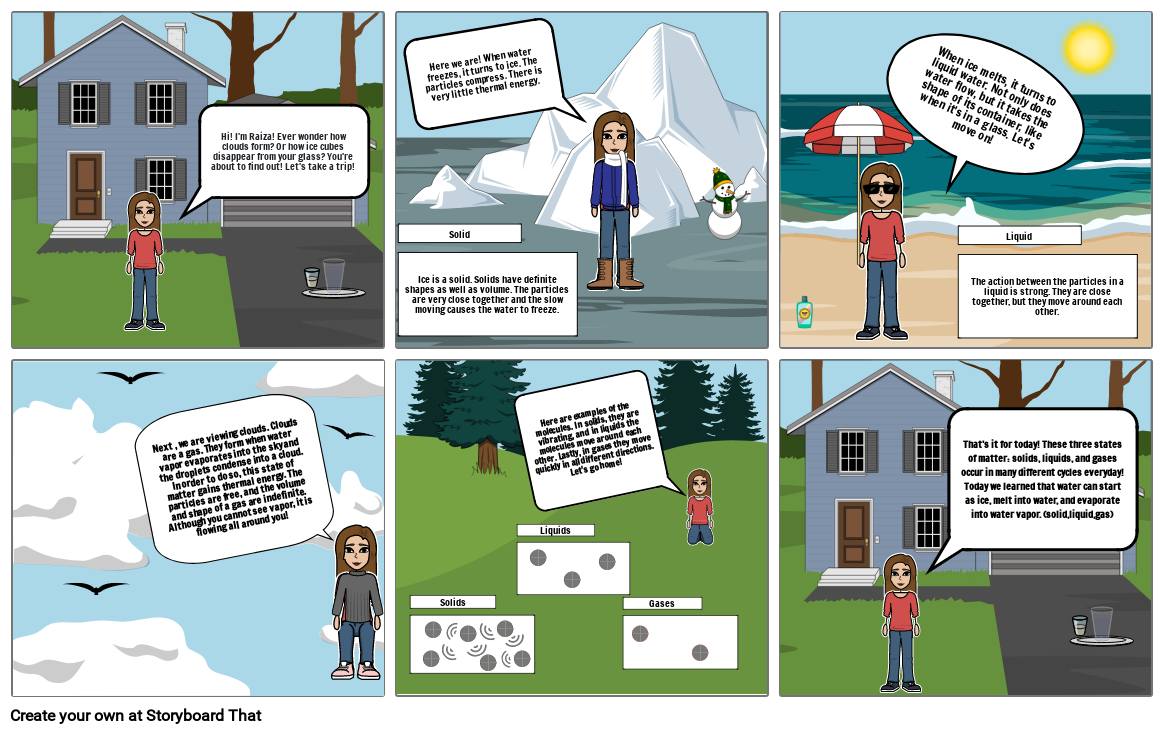
- Hi! I'm Raiza! Ever wonder how clouds form? Or how ice cubes disappear from your glass? You're about to find out! Let's take a trip!
- Solid Ice is a solid. Solids have definite shapes as well as volume. The particles are very close together and the slow moving causes the water to freeze.
- Here we are! When water freezes, it turns to ice. The particles compress. There is very little thermal energy.
- When ice melts, it turns to liquid water. Not only does water flow, but it takes the shape of its container, like when it's in a glass. Let's move on!
- Liquid The action between the particles in a liquid is strong. They are close together, but they move around each other.
- Next , we are viewing clouds. Clouds are a gas. They form when water vapor evaporates into the sky and the droplets condense into a cloud. In order to do so, this state of matter gains thermal energy. The particles are free, and the volume and shape of a gas are indefinite. Although you cannot see vapor, it is flowing all around you!
- Solids
- Liquids
- Here are examples of the molecules. In solids, they are vibrating, and in liquids the molecules move around each other. Lastly, in gases they move quickly in all different directions. Let's go home!
- Gases
- That's it for today! These three states of matter: solids, liquids, and gases occur in many different cycles everyday! Today we learned that water can start as ice, melt into water, and evaporate into water vapor. (solid,liquid,gas)
Más de 30 millones de guiones gráficos creados

