Development of the Atomic Theory Comic
Texto del Guión Gráfico
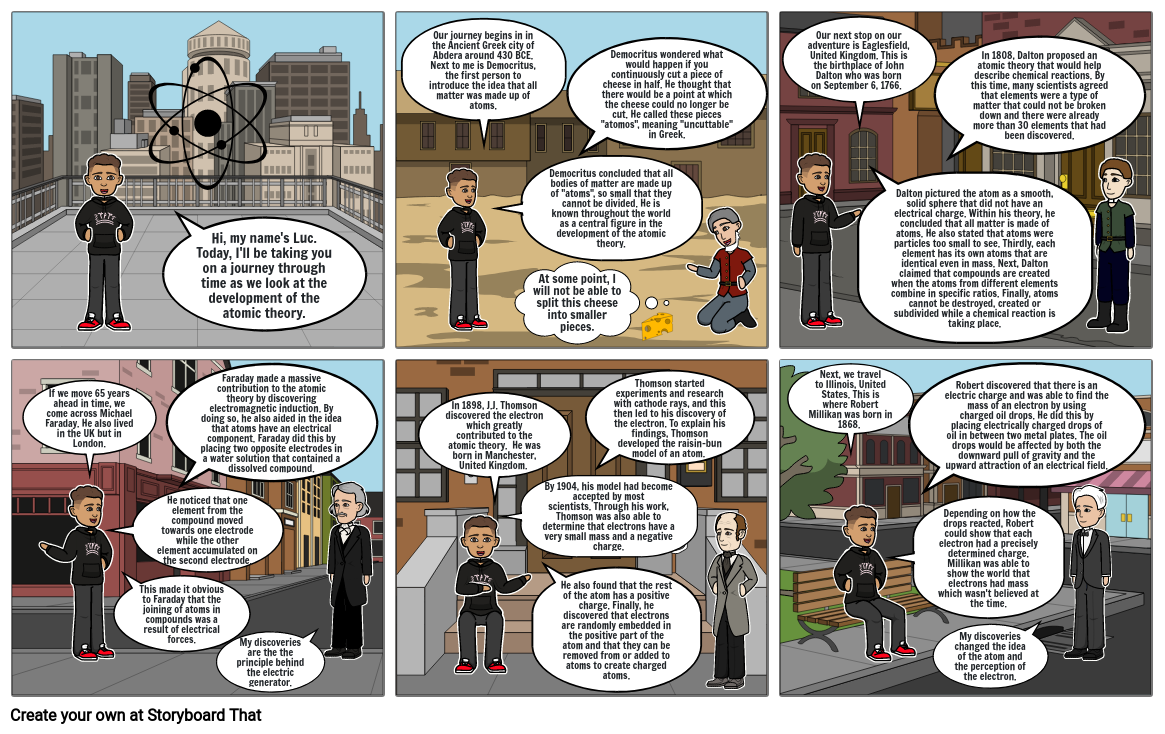
- Hi, my name's Luc. Today, I'll be taking you on a journey through time as we look at the development of the atomic theory.
- Our journey begins in in the Ancient Greek city of Abdera around 430 BCE. Next to me is Democritus, the first person to introduce the idea that all matter was made up of atoms.
- Democritus concluded that all bodies of matter are made up of "atoms", so small that they cannot be divided. He is known throughout the world as a central figure in the development of the atomic theory.
- Democritus wondered what would happen if you continuously cut a piece of cheese in half. He thought that there would be a point at which the cheese could no longer be cut. He called these pieces "atomos", meaning "uncuttable" in Greek.
- At some point, I will not be able to split this cheese into smaller pieces.
- Our next stop on our adventure is Eaglesfield, United Kingdom. This is the birthplace of John Dalton who was born on September 6, 1766.
- Dalton pictured the atom as a smooth, solid sphere that did not have an electrical charge. Within his theory, he concluded that all matter is made of atoms. He also stated that atoms were particles too small to see. Thirdly, each element has its own atoms that are identical even in mass. Next, Dalton claimed that compounds are created when the atoms from different elements combine in specific ratios. Finally, atoms cannot be destroyed, created or subdivided while a chemical reaction is taking place.
- In 1808, Dalton proposed an atomic theory that would help describe chemical reactions. By this time, many scientists agreed that elements were a type of matter that could not be broken down and there were already more than 30 elements that had been discovered.
- If we move 65 years ahead in time, we come across Michael Faraday. He also lived in the UK but in London.
- He noticed that one element from the compound moved towards one electrode while the other element accumulated on the second electrode.
- This made it obvious to Faraday that the joining of atoms in compounds was a result of electrical forces.
- Faraday made a massive contribution to the atomic theory by discovering electromagnetic induction. By doing so, he also aided in the idea that atoms have an electrical component. Faraday did this by placing two opposite electrodes in a water solution that contained a dissolved compound.
- My discoveries are the the principle behind the electric generator.
- In 1898, J.J. Thomson discovered the electron which greatly contributed to the atomic theory. He was born in Manchester, United Kingdom.
- By 1904, his model had become accepted by most scientists. Through his work, Thomson was also able to determine that electrons have a very small mass and a negative charge.
- He also found that the rest of the atom has a positive charge. Finally, he discovered that electrons are randomly embedded in the positive part of the atom and that they can be removed from or added to atoms to create charged atoms.
- Thomson started experiments and research with cathode rays, and this then led to his discovery of the electron. To explain his findings, Thomson developed the raisin-bun model of an atom.
- Next, we travel to Illinois, United States. This is where Robert Millikan was born in 1868.
- Depending on how the drops reacted, Robert could show that each electron had a precisely determined charge. Millikan was able to show the world that electrons had mass which wasn't believed at the time.
- Robert discovered that there is an electric charge and was able to find the mass of an electron by using charged oil drops. He did this by placing electrically charged drops of oil in between two metal plates. The oil drops would be affected by both the downward pull of gravity and the upward attraction of an electrical field.
- My discoveries changed the idea of the atom and the perception of the electron.
Más de 30 millones de guiones gráficos creados

