Quantitative Analysis
Texto del Guión Gráfico
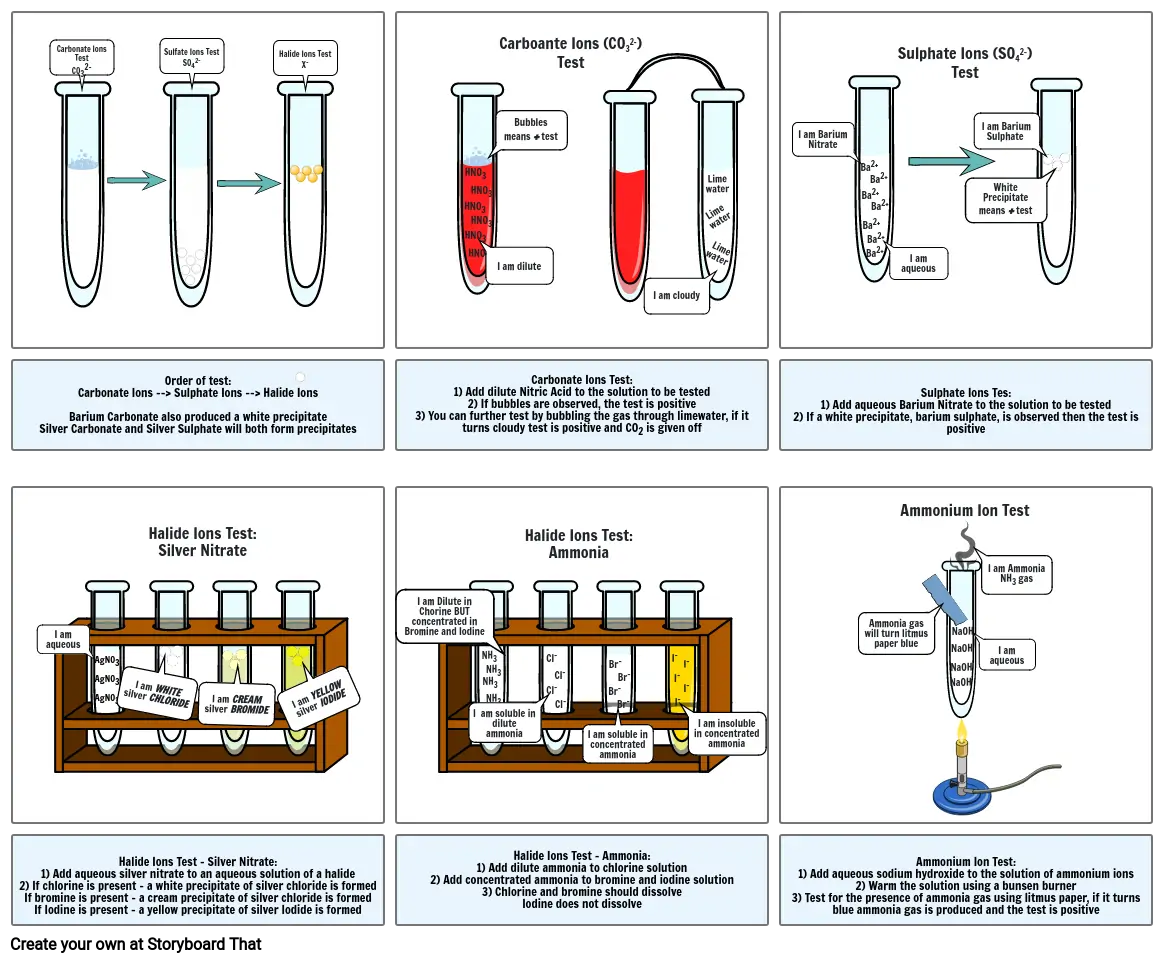
- Carbonate Ions TestCO32-
- Sulfate Ions TestSO42-
- Halide Ions TestX-
- HNO3
- HNO3
- HNO3
- HNO3
- HNO3
- HNO3
- Carboante Ions (CO32-) Test
- I am dilute
- Bubbles means + test
- I am cloudy
- Limewater
- Limewater
- Limewater
- I am Barium Nitrate
- Ba2+
- Ba2+
- Ba2+
- Ba2+
- Ba2+
- Ba2+
- Ba2+
- I am aqueous
- Sulphate Ions (SO42-)Test
- White Precipitate means + test
- I am Barium Sulphate
- Order of test:Carbonate Ions --> Sulphate Ions --> Halide IonsBarium Carbonate also produced a white precipitateSilver Carbonate and Silver Sulphate will both form precipitates
- I am aqueous
- AgNO3
- AgNO3
- AgNO3
- Halide Ions Test:Silver Nitrate
- I am Dilute in Chorine BUT concentrated in Bromine and Iodine
- Carbonate Ions Test:1) Add dilute Nitric Acid to the solution to be tested2) If bubbles are observed, the test is positive3) You can further test by bubbling the gas through limewater, if it turns cloudy test is positive and CO2 is given off
- I am soluble in dilute ammonia
- NH3
- NH3
- NH3
- NH3
- Halide Ions Test:Ammonia
- Cl-
- Cl-
- Cl-
- Cl-
- Br-
- Br-
- Br-
- I-
- I-
- I-
- I-
- Sulphate Ions Tes:1) Add aqueous Barium Nitrate to the solution to be tested2) If a white precipitate, barium sulphate, is observed then the test is positive
-
- Ammonia gas will turn litmus paper blue
- Ammonium Ion Test
-
- NaOH
- NaOH
- NaOH
- NaOH
- I am aqueous
- I am Ammonia NH3 gas
- Halide Ions Test - Silver Nitrate:1) Add aqueous silver nitrate to an aqueous solution of a halide2) If chlorine is present - a white precipitate of silver chloride is formedIf bromine is present - a cream precipitate of silver chloride is formedIf Iodine is present - a yellow precipitate of silver Iodide is formed
- I am WHITE silver CHLORIDE
- I am CREAM silver BROMIDE
- I am YELLOW silver IODIDE
- Halide Ions Test - Ammonia:1) Add dilute ammonia to chlorine solution2) Add concentrated ammonia to bromine and iodine solution3) Chlorine and bromine should dissolveIodine does not dissolve
- I am soluble in concentrated ammonia
- Br-
- I-
- I am insoluble in concentrated ammonia
- Ammonium Ion Test:1) Add aqueous sodium hydroxide to the solution of ammonium ions2) Warm the solution using a bunsen burner3) Test for the presence of ammonia gas using litmus paper, if it turns blue ammonia gas is produced and the test is positive
Más de 30 millones de guiones gráficos creados

