Intermolecular Forces
Texto del Guión Gráfico
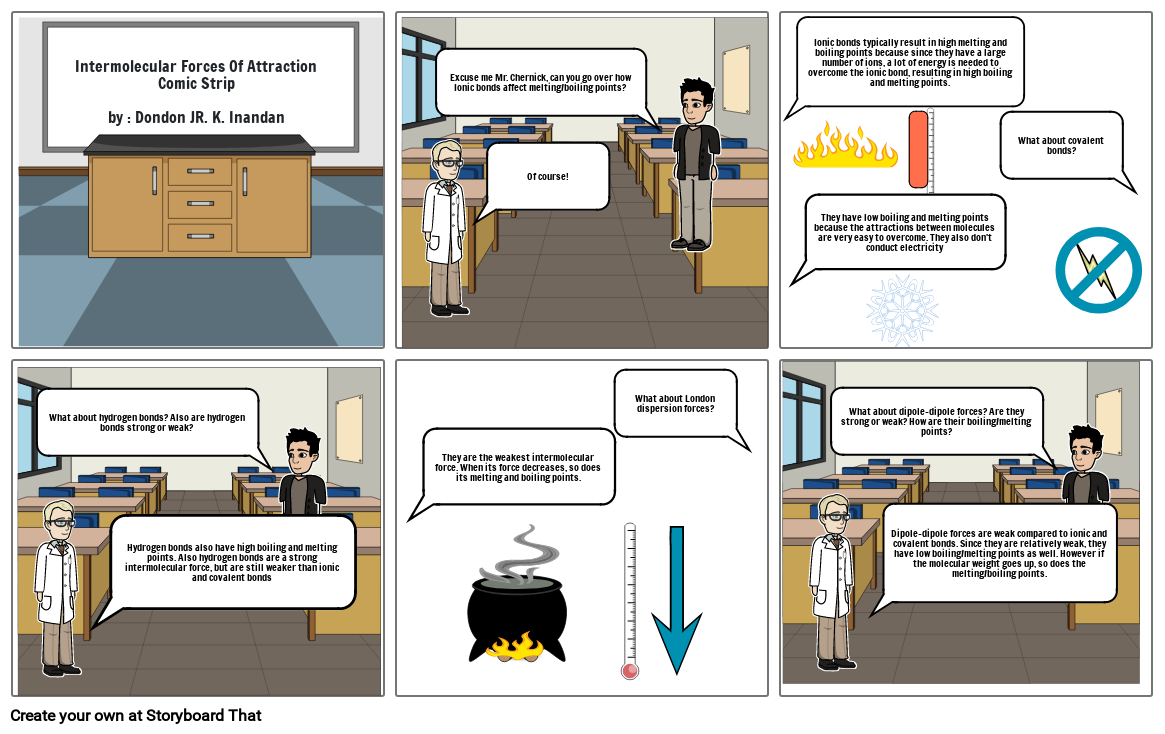
- Intermolecular Forces Of Attraction Comic Stripby : Dondon JR. K. Inandan
- Excuse me Mr. Chernick, can you go over how Ionic bonds affect melting/boiling points?
- Of course!
- Ionic bonds typically result in high melting and boiling points because since they have a large number of ions, a lot of energy is needed to overcome the ionic bond, resulting in high boiling and melting points.
- They have low boiling and melting points because the attractions between molecules are very easy to overcome. They also don't conduct electricity
- What about covalent bonds?
- What about hydrogen bonds? Also are hydrogen bonds strong or weak?
- Hydrogen bonds also have high boiling and melting points. Also hydrogen bonds are a strong intermolecular force, but are still weaker than ionic and covalent bonds
- They are the weakest intermolecular force. When its force decreases, so does its melting and boiling points.
- What about London dispersion forces?
- What about dipole-dipole forces? Are they strong or weak? How are their boiling/melting points?
- Dipole-dipole forces are weak compared to ionic and covalent bonds. Since they are relatively weak, they have low boiling/melting points as well. However if the molecular weight goes up, so does the melting/boiling points.
Más de 30 millones de guiones gráficos creados
¡Sin Descargas, sin Tarjeta de Crédito y sin Necesidad de Iniciar Sesión Para Probar!
