Boyles Law
Storyboard-Text
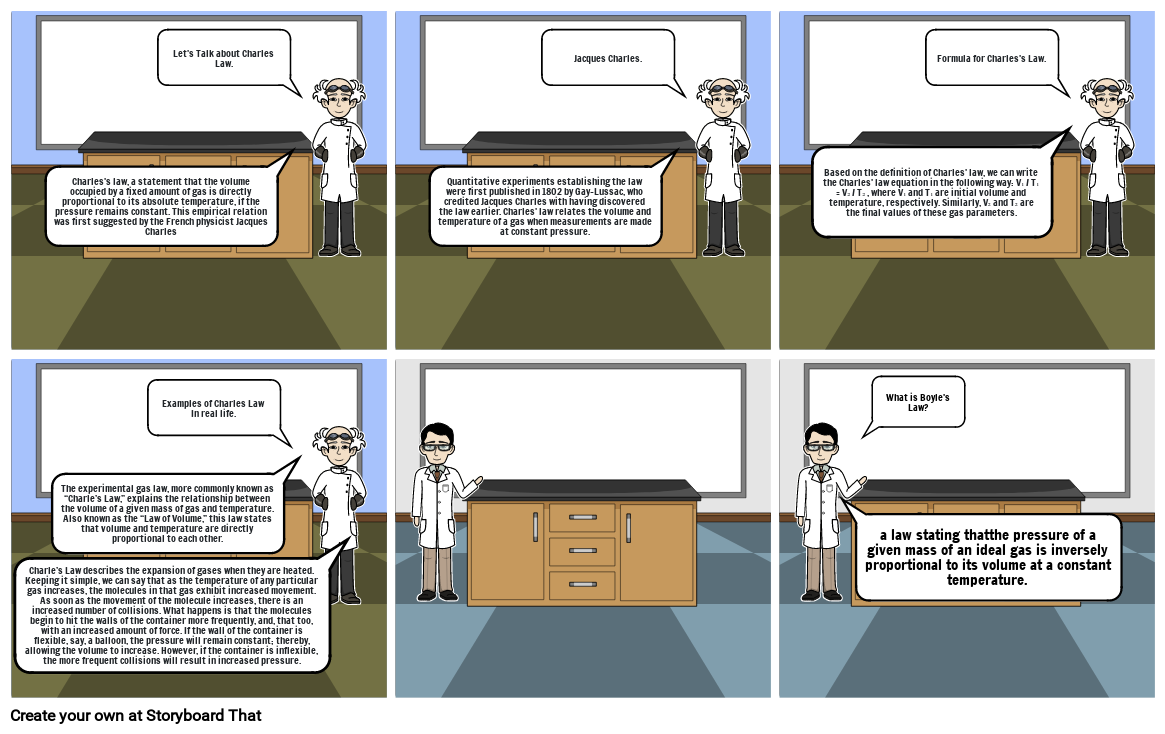
- Charles's law, a statement that the volume occupied by a fixed amount of gas is directly proportional to its absolute temperature, if the pressure remains constant. This empirical relation was first suggested by the French physicist Jacques Charles
- Let's Talk about Charles Law.
- Quantitative experiments establishing the law were first published in 1802 by Gay-Lussac, who credited Jacques Charles with having discovered the law earlier. Charles' law relates the volume and temperature of a gas when measurements are made at constant pressure.
- Jacques Charles.
- Based on the definition of Charles' law, we can write the Charles' law equation in the following way: V₁ / T₁ = V₂ / T₂ , where V₁ and T₁ are initial volume and temperature, respectively. Similarly, V₂ and T₂ are the final values of these gas parameters.
- Formula for Charles's Law.
- Charle’s Law describes the expansion of gases when they are heated. Keeping it simple, we can say that as the temperature of any particular gas increases, the molecules in that gas exhibit increased movement. As soon as the movement of the molecule increases, there is an increased number of collisions. What happens is that the molecules begin to hit the walls of the container more frequently, and, that too, with an increased amount of force. If the wall of the container is flexible, say, a balloon, the pressure will remain constant; thereby, allowing the volume to increase. However, if the container is inflexible, the more frequent collisions will result in increased pressure.
- The experimental gas law, more commonly known as “Charle’s Law,” explains the relationship between the volume of a given mass of gas and temperature. Also known as the “Law of Volume,” this law states that volume and temperature are directly proportional to each other.
- Examples of Charles Law In real life.
- a law stating thatthe pressure of a given mass of an ideal gas is inversely proportional to its volume at a constant temperature.
- What is Boyle's Law?
Über 30 Millionen erstellte Storyboards

