Atomic theory
Storyboard-Text
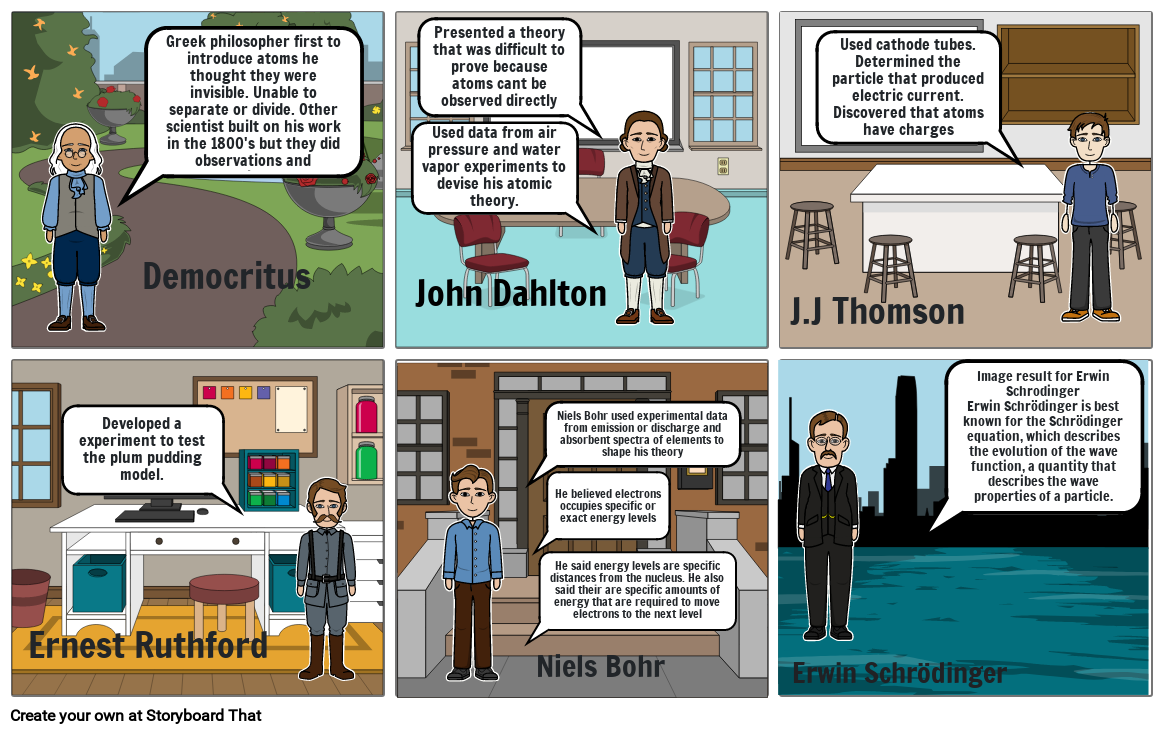
- Greek philosopher first to introduce atoms he thought they were invisible. Unable to separate or divide. Other scientist built on his work in the 1800's but they did observations and experiments.
- Democritus
- John Dahlton
- Used data from air pressure and water vapor experiments to devise his atomic theory.
- Presented a theory that was difficult to prove because atoms cant be observed directly
- J.J Thomson
- Used cathode tubes. Determined the particle that produced electric current. Discovered that atoms have charges
- Ernest Ruthford
- Developed a experiment to test the plum pudding model.
- He said energy levels are specific distances from the nucleus. He also said their are specific amounts of energy that are required to move electrons to the next level
- Niels Bohr used experimental data from emission or discharge and absorbent spectra of elements to shape his theory
- He believed electrons occupies specific or exact energy levels
- Niels Bohr
- Erwin Schrödinger
- Image result for Erwin SchrodingerErwin Schrödinger is best known for the Schrödinger equation, which describes the evolution of the wave function, a quantity that describes the wave properties of a particle.
Über 30 Millionen erstellte Storyboards

