Atomic Theories Story Board
Storyboard Text
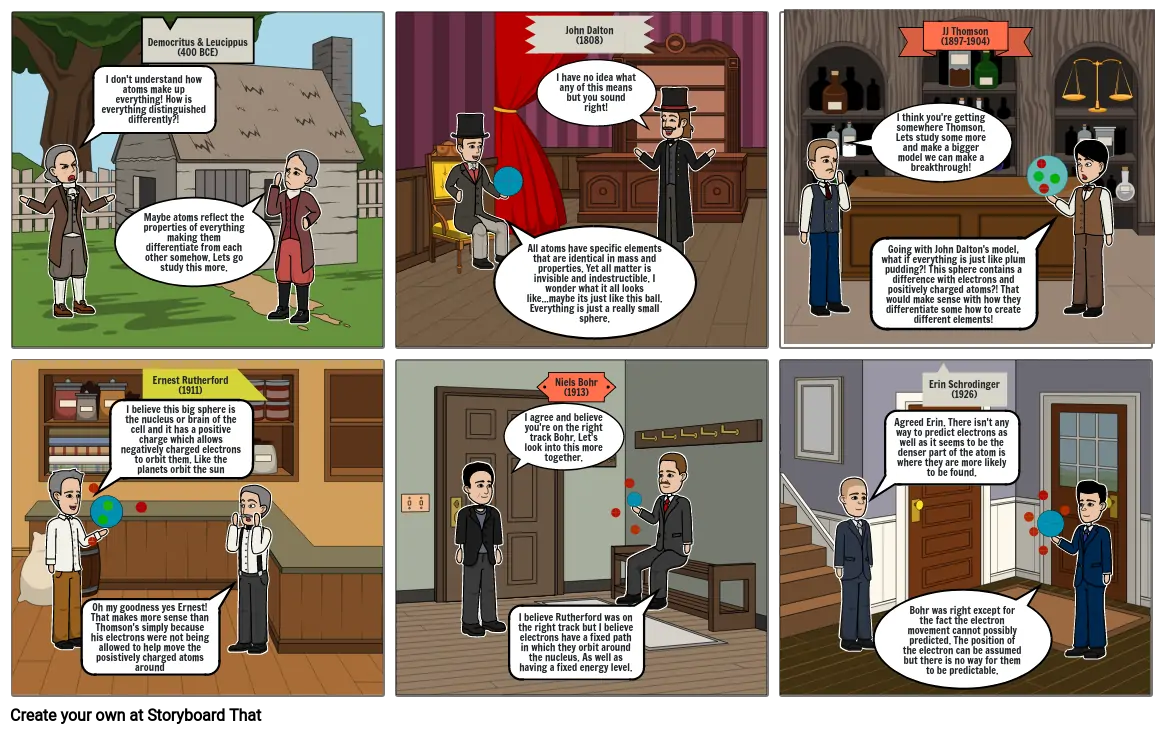
- I don't understand how atoms make up everything! How is everything distinguished differently?!
- Maybe atoms reflect the properties of everything making them differentiate from each other somehow. Lets go study this more.
- Democritus & Leucippus (400 BCE)
- All atoms have specific elements that are identical in mass and properties. Yet all matter is invisible and indestructible. I wonder what it all looks like...maybe its just like this ball. Everything is just a really small sphere.
- John Dalton (1808)
- I have no idea what any of this means but you sound right!
- I think you're getting somewhere Thomson. Lets study some more and make a bigger model we can make a breakthrough!
- Going with John Dalton's model, what if everything is just like plum pudding?! This sphere contains a difference with electrons and positively charged atoms?! That would make sense with how they differentiate some how to create different elements!
- JJ Thomson (1897-1904)
- Oh my goodness yes Ernest! That makes more sense than Thomson's simply because his electrons were not being allowed to help move the posistively charged atoms around
- I believe this big sphere is the nucleus or brain of the cell and it has a positive charge which allows negatively charged electrons to orbit them. Like the planets orbit the sun
- Ernest Rutherford (1911)
- I agree and believe you're on the right track Bohr. Let's look into this more together.
- I believe Rutherford was on the right track but I believe electrons have a fixed path in which they orbit around the nucleus. As well as having a fixed energy level.
- Niels Bohr (1913)
- Agreed Erin. There isn't any way to predict electrons as well as it seems to be the denser part of the atom is where they are more likely to be found.
- Bohr was right except for the fact the electron movement cannot possibly predicted. The position of the electron can be assumed but there is no way for them to be predictable.
- Erin Schrodinger (1926)
Vytvořeno více než 30 milionů Storyboardů

