Part 1 Chemistry 2021
Текст на Статията
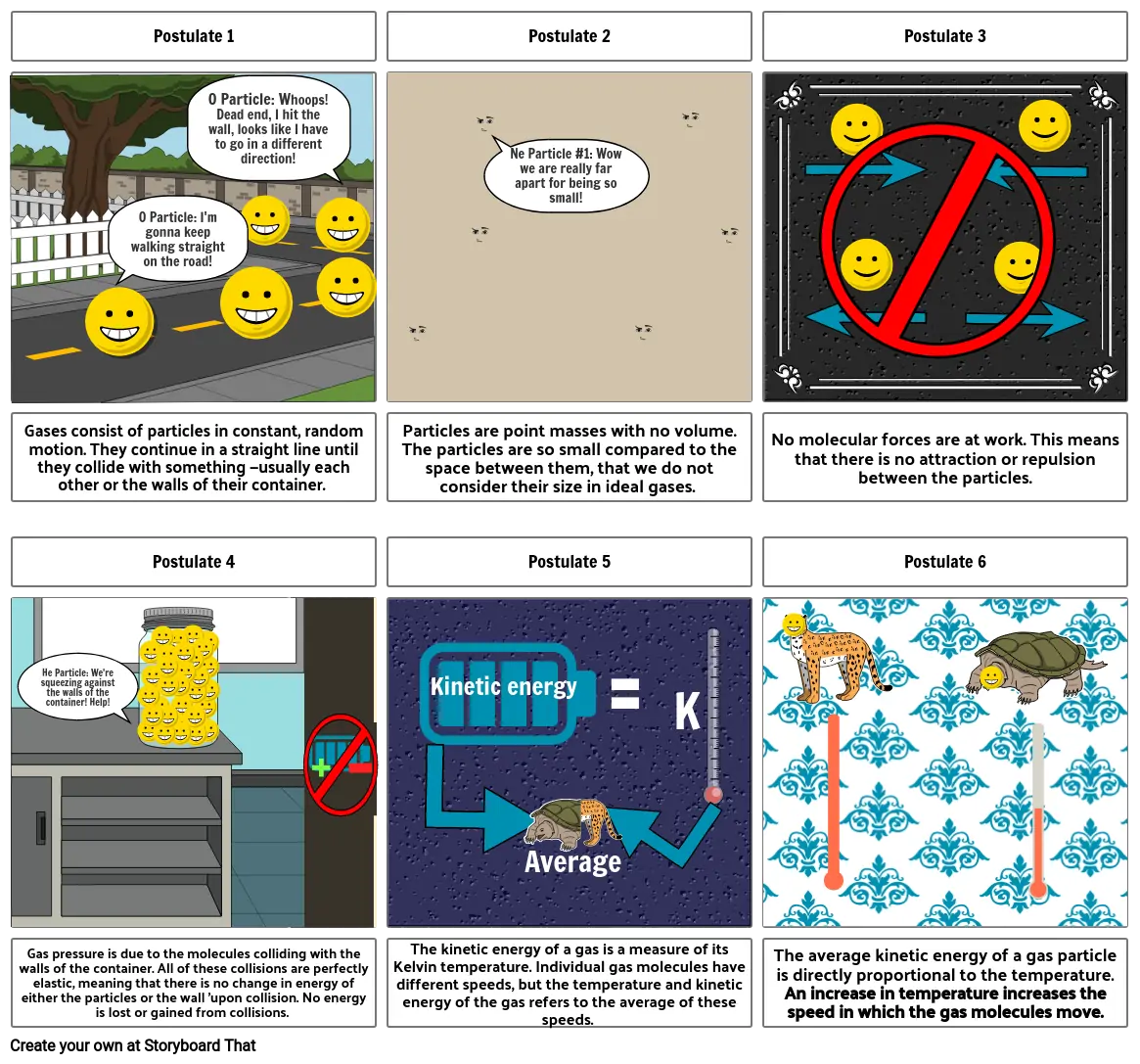
- Postulate 1
- O Particle: I'm gonna keep walking straight on the road!
- O Particle: Whoops! Dead end, I hit the wall, looks like I have to go in a different direction!
- Postulate 2
- Ne Particle #1: Wow we are really far apart for being so small!
- Postulate 3
- Gases consist of particles in constant, random motion. They continue in a straight line until they collide with something —usually each other or the walls of their container.
- Postulate 4
- He Particle: We're squeezing against the walls of the container! Help!
- Kinetic energy
- Particles are point masses with no volume. The particles are so small compared to the space between them, that we do not consider their size in ideal gases.
- Postulate 5
- =
- K
- No molecular forces are at work. This means that there is no attraction or repulsion between the particles.
- Postulate 6
- Gas pressure is due to the molecules colliding with the walls of the container. All of these collisions are perfectly elastic, meaning that there is no change in energy of either the particles or the wall 'upon collision. No energy is lost or gained from collisions.
- The kinetic energy of a gas is a measure of its Kelvin temperature. Individual gas molecules have different speeds, but the temperature and kinetic energy of the gas refers to the average of these speeds.
- Average
- The average kinetic energy of a gas particle is directly proportional to the temperature. An increase in temperature increases the speed in which the gas molecules move.
Над 30 милиона създадени разкадровки

