science comic strip
Текст на Статията
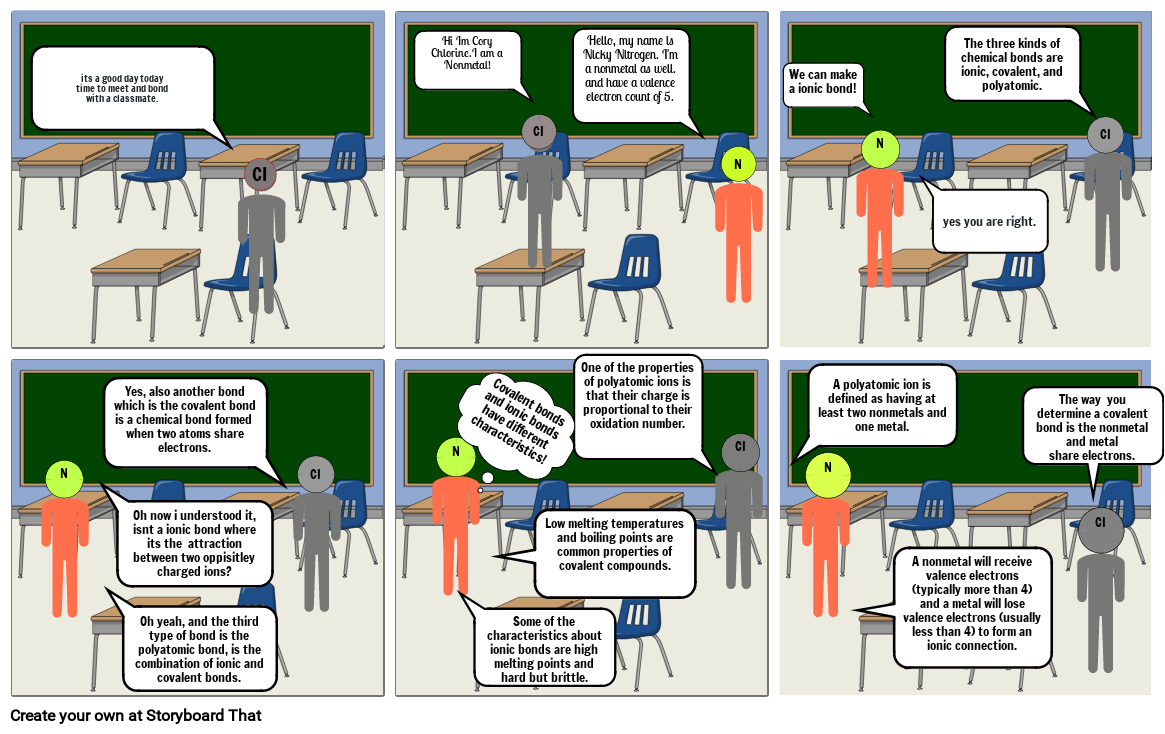
- its a good day todaytime to meet and bondwith a classmate.
- CI
- Hi Im Cory Chlorine.I am a Nonmetal!
- CI
- Hello, my name is Nicky Nitrogen. I'm a nonmetal as well.and have a valence electron count of 5.
- N
- We can make a ionic bond!
- N
- yes you are right.
- The three kinds of chemical bonds are ionic, covalent, and polyatomic.
- CI
- N
- Oh now i understood it, isnt a ionic bond where its the  attraction between two oppisitley charged ions?
- Yes, also another bond which is the covalent bond is a chemical bond formed when two atoms share electrons.
- Oh yeah, and the third type of bond is the polyatomic bond, is the combination of ionic and covalent bonds.
- CI
- N
- Covalent bonds and ionic bonds have different characteristics!
- Some of the characteristics about ionic bonds are high melting points and hard but brittle.
- Low melting temperatures and boiling points are common properties of covalent compounds.
- One of the properties of polyatomic ions is that their charge is proportional to their oxidation number.
- CI
- A polyatomic ion is defined as having at least two nonmetals and one metal.
- N
- A nonmetal will receive valence electrons (typically more than 4) and a metal will lose valence electrons (usually less than 4) to form an ionic connection.
- The way  you determine a covalent bond is the nonmetal and metal share electrons.
- CI
Над 30 милиона създадени разкадровки

