atomic theory history
Текст на Статията
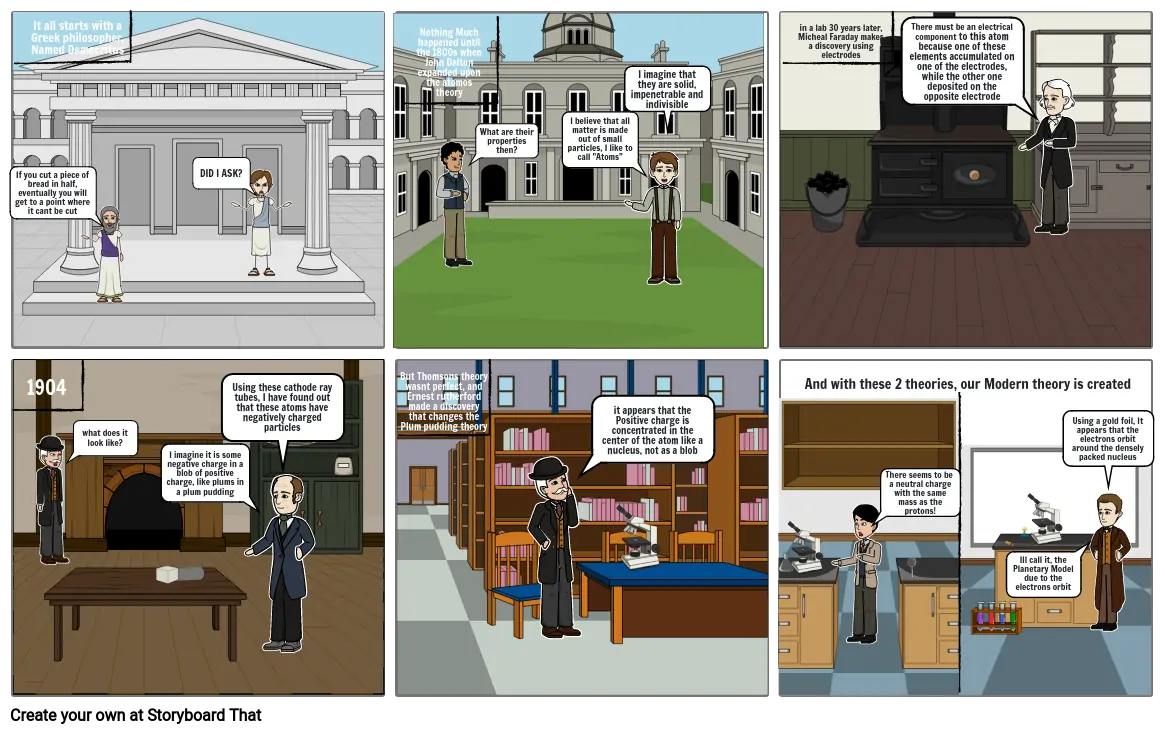
- If you cut a piece of bread in half, eventually you will get to a point where it cant be cut
- It all starts with a Greek philosopher, Named Democritus
- DID I ASK?
- Nothing Much happened until the 1800s when John Dalton expanded upon the atomos theory
- What are their properties then?
- I believe that all matter is made out of small particles, I like to call "Atoms"
- I imagine that they are solid, impenetrable and indivisible
- in a lab 30 years later, Micheal Faraday makes a discovery using electrodes
- There must be an electrical component to this atom because one of these elements accumulated on one of the electrodes, while the other one deposited on the opposite electrode
- 1904
- what does it look like?
- I imagine it is some negative charge in a blob of positive charge, like plums in a plum pudding
- Using these cathode ray tubes, I have found out that these atoms have negatively charged particles
- But Thomsons theory wasnt perfect, and Ernest rutherford made a discovery that changes the Plum pudding theory
- it appears that the Positive charge is concentrated in the center of the atom like a nucleus, not as a blob
- And with these 2 theories, our Modern theory is created
- There seems to be a neutral charge with the same mass as the protons!
- Ill call it, the Planetary Model due to the electrons orbit
- Using a gold foil, It appears that the electrons orbit around the densely packed nucleus
Над 30 милиона създадени разкадровки

