Ionic bonding comic strip
نص القصة المصورة
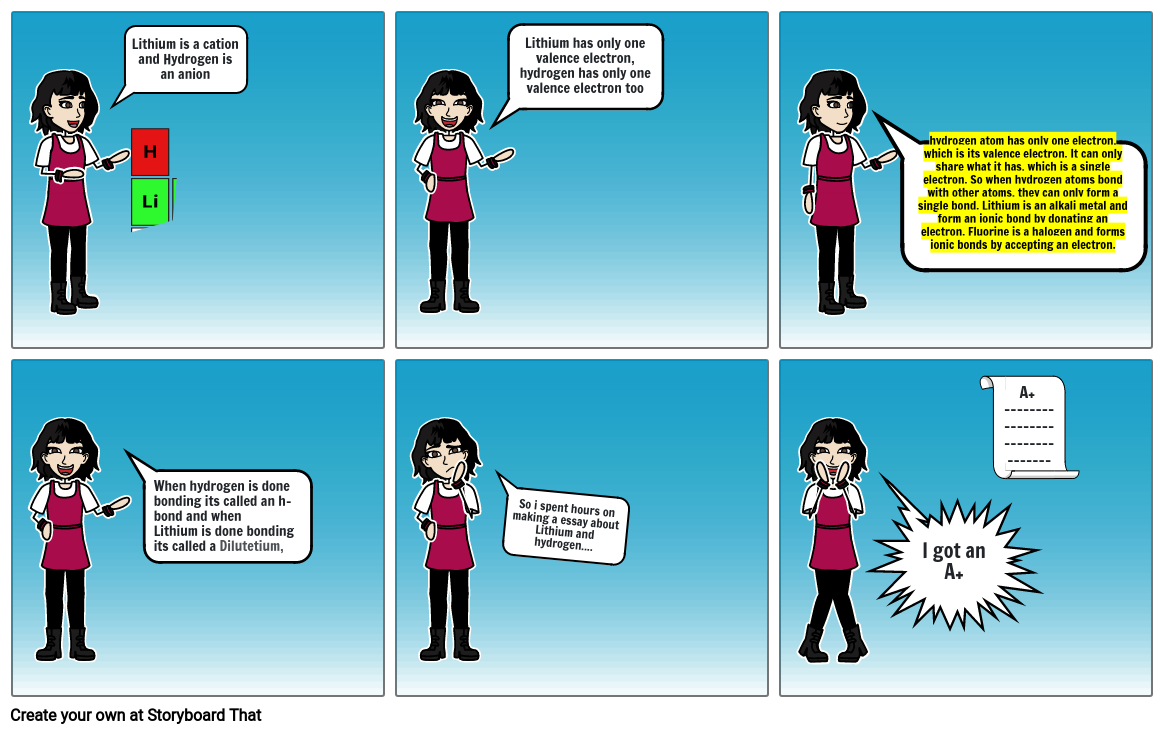
- Lithium is a cation and Hydrogen is an anion
- Lithium has only one valence electron, hydrogen has only one valence electron too
- hydrogen atom has only one electron, which is its valence electron. It can only share what it has, which is a single electron. So when hydrogen atoms bond with other atoms, they can only form a single bond. Lithium is an alkali metal and form an ionic bond by donating an electron. Fluorine is a halogen and forms ionic bonds by accepting an electron.
- When hydrogen is done bonding its called an h-bond and when Lithium is done bonding its called a Dilutetium,
- So i spent hours on making a essay about Lithium and hydrogen....
- I got an A+
- A+ -------------------------------
تم إنشاء أكثر من 30 مليون من القصص المصورة
لا توجد تنزيلات ولا بطاقة ائتمان ولا حاجة إلى تسجيل الدخول للمحاولة!



