BOYLE'S LAW
نص القصة المصورة
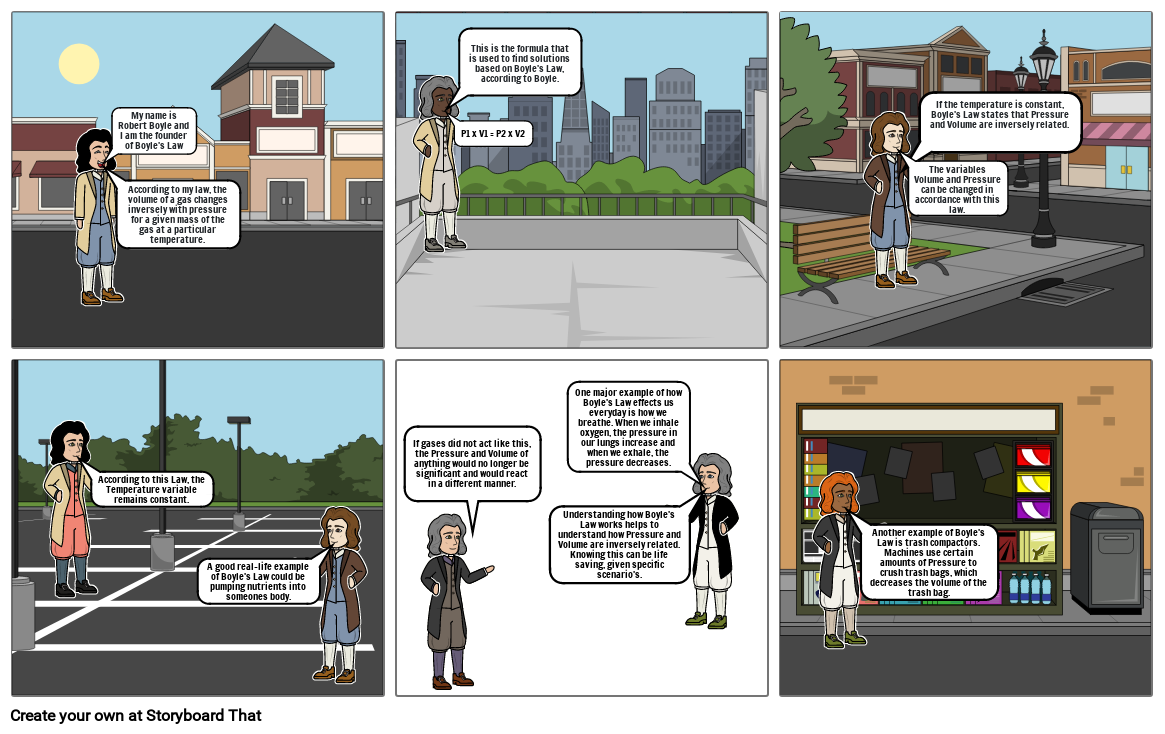
- According to my law, the volume of a gas changes inversely with pressure for a given mass of the gas at a particular temperature.
- My name is Robert Boyle and I am the founder of Boyle's Law
- This is the formula that is used to find solutions based on Boyle's Law, according to Boyle.
- P1 x V1 = P2 x V2
- The variables Volume and Pressure can be changed in accordance with this law.
- If the temperature is constant, Boyle's Law states that Pressure and Volume are inversely related.
- According to this Law, the Temperature variable remains constant.
- A good real-life example of Boyle's Law could be pumping nutrients into someones body.
- If gases did not act like this, the Pressure and Volume of anything would no longer be significant and would react in a different manner.
- Understanding how Boyle's Law works helps to understand how Pressure and Volume are inversely related. Knowing this can be life saving, given specific scenario's.
- One major example of how Boyle's Law effects us everyday is how we breathe. When we inhale oxygen, the pressure in our lungs increase and when we exhale, the pressure decreases.
- Another example of Boyle's Law is trash compactors. Machines use certain amounts of Pressure to crush trash bags, which decreases the volume of the trash bag.
تم إنشاء أكثر من 30 مليون من القصص المصورة

