Unknown Story
نص القصة المصورة
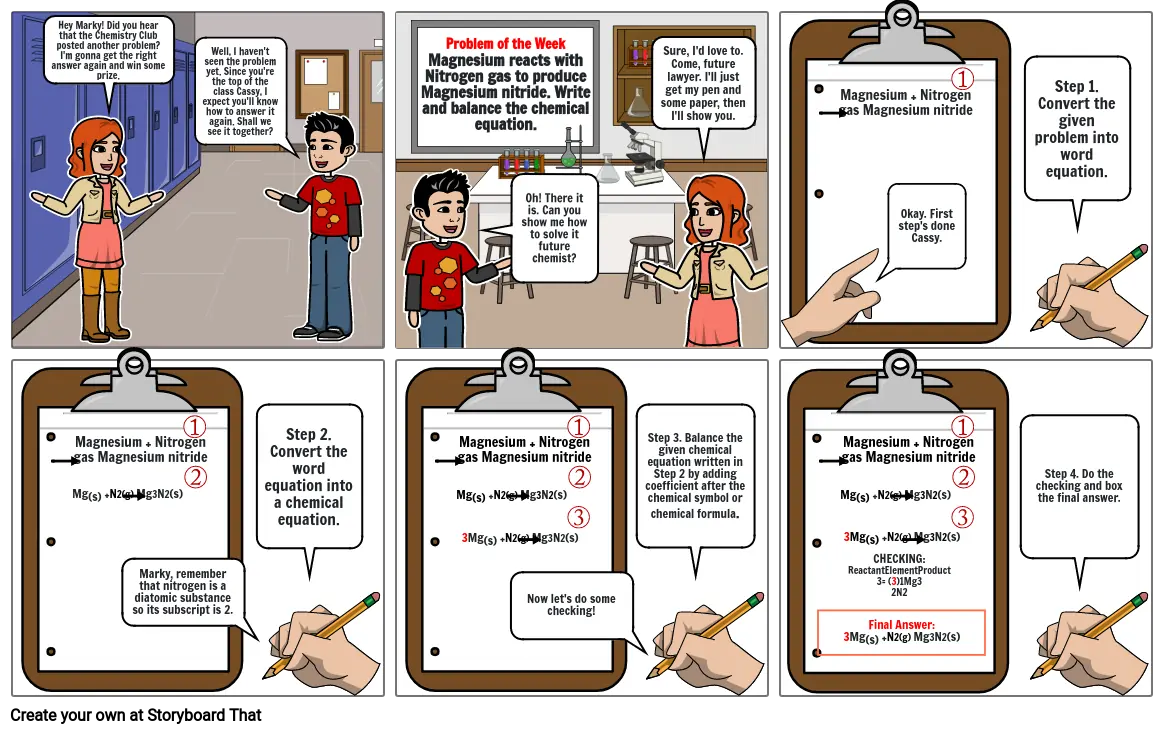
- Hey Marky! Did you hear that the Chemistry Club posted another problem? I'm gonna get the right answer again and win some prize.
- Well, I haven't seen the problem yet. Since you're the top of the class Cassy, I expect you'll know how to answer it again. Shall we see it together?
- Problem of the WeekMagnesium reacts with Nitrogen gas to produce Magnesium nitride. Write and balance the chemical equation.
- Oh! There it is. Can you show me how to solve it future chemist?
- Sure, I'd love to. Come, future lawyer. I'll just get my pen and some paper, then I'll show you.
- Magnesium + Nitrogen gas Magnesium nitride
- Okay. First step's done Cassy.
- Step 1. Convert the given problem into word equation.
- Mg(s) +N2(g) Mg3N2(s)
- Magnesium + Nitrogen gas Magnesium nitride
- Marky, remember that nitrogen is a diatomic substance so its subscript is 2.
- Step 2. Convert the word equation into a chemical equation.
- Mg(s) +N2(g) Mg3N2(s)
- 3Mg(s) +N2(g) Mg3N2(s)
- Magnesium + Nitrogen gas Magnesium nitride
- Now let's do some checking!
- Step 3. Balance the given chemical equation written in Step 2 by adding coefficient after the chemical symbol or chemical formula.
- CHECKING:ReactantElementProduct3= (3)1Mg32N2
- Mg(s) +N2(g) Mg3N2(s)
- 3Mg(s) +N2(g) Mg3N2(s)
- Final Answer:3Mg(s) +N2(g) Mg3N2(s)
- Magnesium + Nitrogen gas Magnesium nitride
- Step 4. Do the checking and box the final answer.
تم إنشاء أكثر من 30 مليون من القصص المصورة

